
Report ID: SQMIG35H2339

Report ID: SQMIG35H2339
sales@skyquestt.com
USA +1 351-333-4748

Report ID:
SQMIG35H2339 |
Region:
Global |
Published Date: May, 2025
Pages:
187
|Tables:
67
|Figures:
67



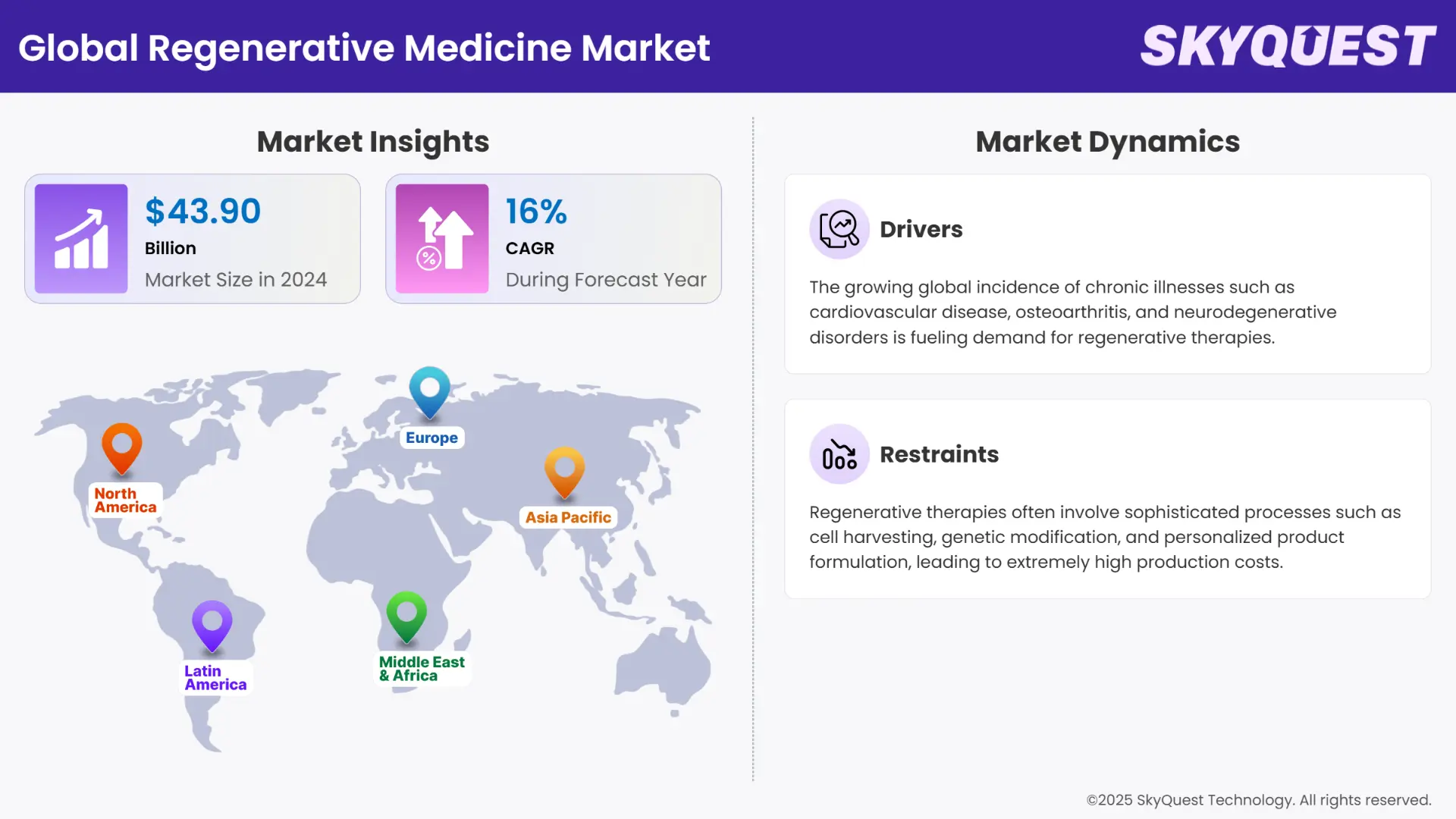
Global Regenerative Medicine Market size was valued at USD 43.90 Billion in 2024 and is poised to grow from USD 50.92 Billion in 2025 to USD 166.95 Billion by 2033, growing at a CAGR of 16% in the forecast period 16%.
The regenerative medicine market is transforming modern healthcare by offering advanced therapeutic solutions aimed at repairing, replacing, or regenerating damaged tissues and organs. It encompasses a broad range of technologies, including stem cell therapy, gene therapy, tissue engineering, and biomaterials. These innovations are finding increasing application in treating chronic diseases, orthopedic conditions, cardiovascular disorders, and neurological impairments. Rising investment from both public and private sectors is fueling R&D activities across academia, biotechnology firms, and pharmaceutical giants. Moreover, growing awareness among patients and practitioners about the long-term benefits of regenerative treatments is encouraging early adoption. With aging populations and a global shift toward personalized medicine, the market is well-positioned to play a central role in the future of disease management and recovery.
Several compelling factors are driving the expansion of the regenerative medicine market. The rising global prevalence of chronic and degenerative diseases is creating urgent demand for more effective and less invasive treatments. Technological advancements in stem cell biology, tissue engineering, and bioprinting have accelerated the development of novel therapies, increasing their clinical applicability. Government support through funding initiatives, favorable regulatory frameworks, and fast-track approvals are further encouraging innovation. Additionally, strategic partnerships between biotech companies and research institutions are boosting product development and commercialization. The growing success of cell and gene therapies in clinical trials, along with increasing FDA and EMA approvals, is building investor confidence. Heightened focus on personalized and precision medicine also aligns well with regenerative medicine’s targeted therapeutic potential.
Despite its promise, the regenerative medicine market faces several critical challenges that could hinder growth. High development costs and complex manufacturing processes significantly elevate product prices, limiting access for many patients and healthcare systems. Regulatory and ethical hurdles surrounding stem cell sourcing, genetic modification, and long-term safety pose ongoing concerns, especially in jurisdictions with evolving legislation. Clinical translation from laboratory success to real-world efficacy remains a bottleneck, with many therapies still in early trial phases. Moreover, insufficient infrastructure and skilled personnel in emerging markets restrict widespread implementation. Concerns regarding immune rejection, tumorigenicity, and durability of therapeutic effects also raise caution. Reimbursement uncertainties and a lack of standardized evaluation criteria for regenerative products further complicate adoption and scalability across global regenerative medicine market.
Artificial intelligence (AI) is revolutionizing the regenerative medicine market by enhancing research efficiency, precision, and scalability. AI algorithms can analyze vast datasets to identify patterns and predict outcomes, accelerating the development of personalized therapies. In stem cell research, AI assists in optimizing culture conditions and predicting differentiation pathways, reducing time and cost. Additionally, AI-driven models aid in tissue engineering by simulating complex biological systems, facilitating the design of functional tissues and organs. These advancements not only expedite the translation of regenerative therapies from bench to bedside but also improve patient outcomes by enabling more targeted and effective treatments.
A notable recent development is the collaboration between Wipro and Pandorum Technologies to integrate AI into regenerative medicine. This partnership focuses on developing bio-engineered tissues, such as liquid corneas, using Wipro's AI platform, Holmes, to predict therapeutic outcomes and optimize clinical study designs. By leveraging AI, the collaboration aims to shorten time-to-market and enhance patient outcomes in regenerative therapies. This initiative exemplifies how AI can be harnessed to accelerate innovation and improve the efficacy of regenerative medicine.
To get more insights on this market click here to Request a Free Sample Report
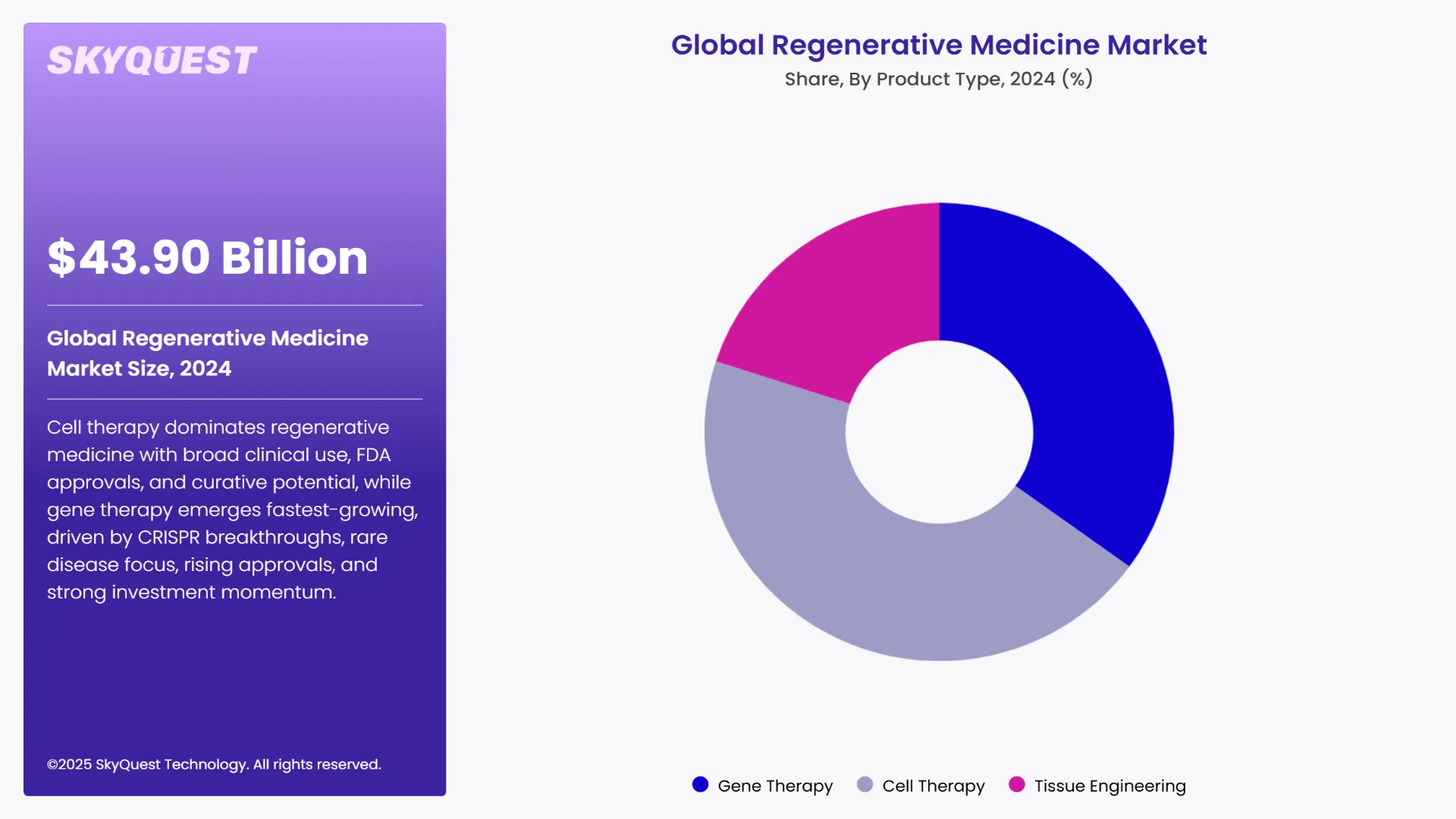
Global Regenerative Medicine Market is segmented by Product Type, Application and region. Based on Product Type, the market is segmented into Gene Therapy, Cell Therapy and Tissue Engineering. Based on Application, the market is segmented into Orthopedic and Dental, Cardiology, Wound Healing, Metabolism and Inflammatory, Immunology and Oncology, Neurology and Others. Based on region, the market is segmented into North America, Europe, Asia Pacific, Latin America and Middle East & Africa.
As per the 2024 global regenerative medicine market analysis, the cell therapy segment led the market by holding the largest share. This dominance is primarily driven by the wide-ranging applications of cell-based treatments across diseases such as cancer, autoimmune disorders, and orthopedic conditions. Cell therapies like CAR-T cells and mesenchymal stem cells have gained numerous FDA approvals, established strong clinical credibility and expanded patient access worldwide. The year 2024 witnessed considerable growth in this segment, supported by ongoing innovation and expanding treatment pipelines. These therapies have transformed traditional treatment methods by offering personalized and curative solutions.
On the other hand, the regenerative medicine market is growing at a sustainable CAGR of around 12%, driven by the increasing acceptance of cell therapies. Based on various research reports, the conversion rates and patient outcomes have improved substantially with cell therapy adoption. This suggests that the market will continue to shift towards these advanced, targeted solutions, generating higher value with optimized treatment efficacy and reduced side effects.
As per the 2024 global regenerative medicine market analysis for Regenerative Medicine, the gene therapy segment is the fastest-growing product type. This rapid growth is primarily fueled by breakthroughs in gene editing technologies such as CRISPR and an increasing number of regulatory approvals for novel gene-based treatments targeting rare genetic disorders and various cancers. These advancements have transformed gene therapy from a niche experimental option into a viable treatment alternative with curative potential. The year 2024 witnessed significant investment and clinical trial activities focusing on gene therapies, accelerating market adoption and commercial availability.
For instance, in June 2024, the FDA approved Gilead Sciences’ gene therapy for a rare cerebral genetic condition, marking a milestone in neurological disorder treatments and boosting investor confidence in gene therapy innovations.
To get detailed segments analysis, Request a Free Sample Report
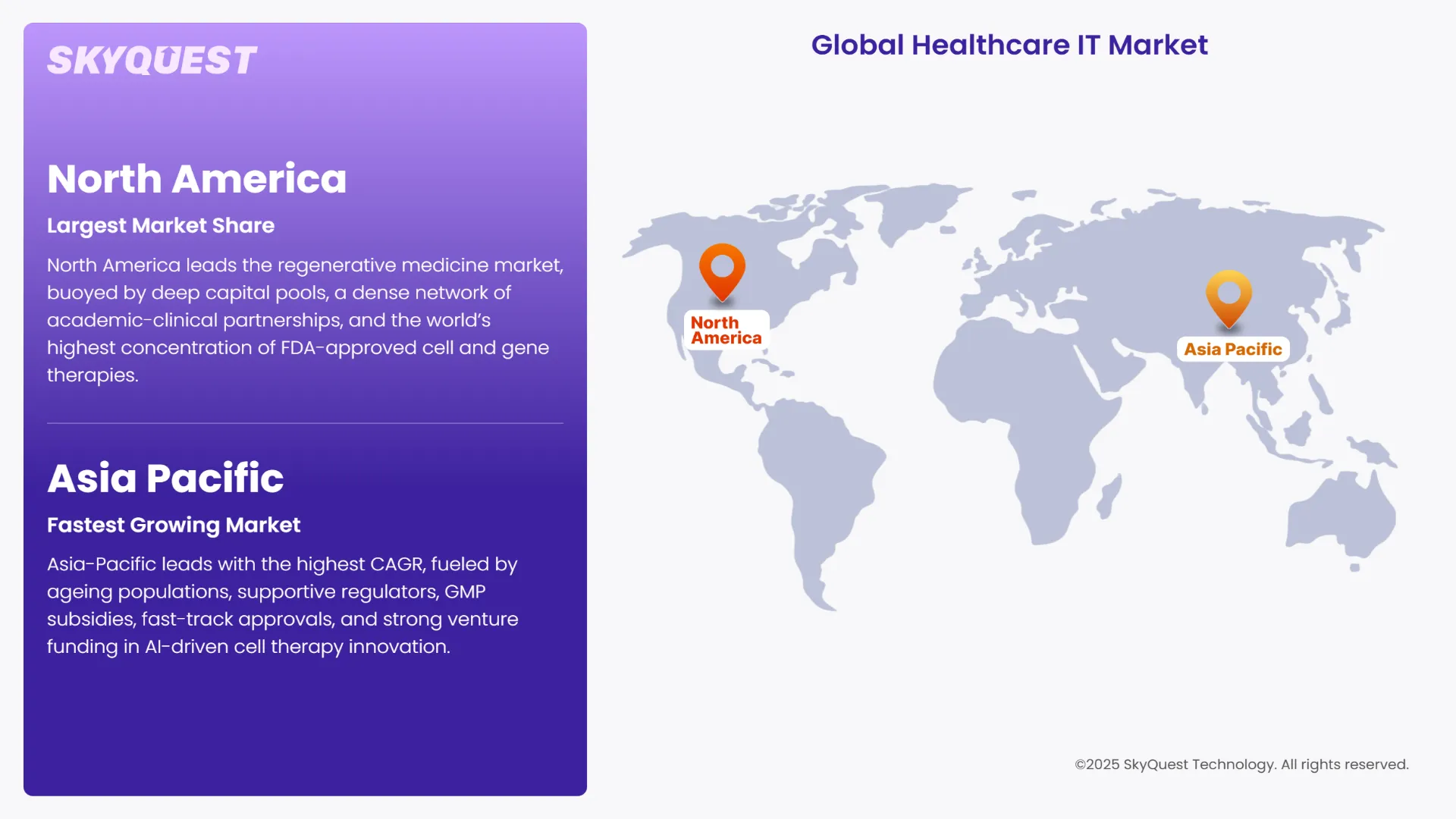
North America leads the regenerative medicine market, buoyed by deep capital pools, a dense network of academic-clinical partnerships, and the world’s highest concentration of FDA-approved cell and gene therapies. Robust venture financing, fast-track regulatory pathways such as the U.S. RMAT designation, and extensive biomanufacturing capacity in hubs like Boston and San Diego sustain rapid product rollouts. Canada’s federal R&D tax credits and newly announced incubation grants further widen the region’s innovation pipeline. Well-established reimbursement channels, large ageing populations, and strong payer acceptance of one-time curative treatments keep demand high, making North America the undisputed revenue engine of the global market.
The United States Regenerative Medicine Market
The U.S. continues to dominate thanks to a surge in late-phase trials and landmark approvals. In February 2025, the FDA cleared a first-in-class CRISPR-edited autologous T-cell therapy for advanced melanoma under Project Orbis, trimming review time to seven months and catalyzing follow-on funding rounds across biotech clusters. Simultaneously, BARDA committed USD 350 million to expand domestic viral-vector manufacturing, ensuring supply-chain resilience. Private investors matched federal enthusiasm, with 1Q 2025 venture funding for U.S. regenerative startups topping USD 4 billion, a 28 % YoY jump. These moves reinforce America’s leadership in clinical translation, scale-up, and commercialization.
Canada Regenerative Medicine Market
Canada’s market momentum is underpinned by supportive policy and strong academic spin-outs. In April 2025, the Stem Cell Network partnered with Capital BioVentures to launch a CAD 25 million fund plus a new Incubation Award Program that directly finances early-stage regenerative startups and provides wet-lab space in Vancouver and Toronto. The federal Strategic Innovation Fund also earmarked CAD 100 million to build a national cell-therapy manufacturing centre, lowering production costs and anchoring talent domestically. These initiatives, alongside streamlined Health Canada Advanced Therapeutic Pathway approvals, position Canada as a complementary growth engine to the U.S., attracting cross-border trials and licensing deals.
Asia-Pacific posts the highest CAGR, driven by ageing demographics, proactive regulators, and aggressive infrastructure spending. Governments subsidize GMP facilities and offer conditional approvals that hasten market entry, while regional venture funds pour capital into AI-enabled cell-therapy discovery. Expanding medical-tourism corridors and competitive treatment pricing further amplify uptake. Japan and South Korea anchor the region’s boom with clear legal frameworks and reimbursement pilots, while optional giants China and India add volume through populous patient pools and expanding hospital networks. Collectively, these dynamics propel Asia-Pacific to eclipse double-digit growth through 2030.
Japan Regenerative Medicine Market
Japan couples’ generous reimbursement with cutting-edge logistics. As of March 2025, the country operates more than 150 specialized cold-chain hubs dedicated to cell and gene therapies, slashing last-mile costs and turnaround times. Concurrent regulatory reforms are easing secondary use of partially anonymized genomic data, unlocking AI-driven therapy optimization while upholding privacy norms. SoftBank’s joint venture with Tempus exemplifies this shift: the partnership plans to feed 10 million de-identified patient records into machine-learning models to fast-track precision-engineered tissue grafts. Such infrastructure-plus-data synergy cements Japan’s role as the regional innovation testbed.
South Korea Regenerative Medicine Market
South Korea became APAC’s regulatory pace-setter with its February 21 2025 Regenerative Medicine Law, a comprehensive act that lets patients with serious or rare conditions access yet-unapproved cell and gene therapies under strict safety monitoring. The law also mandates centralized adverse-event tracking and guarantees five-year R&D tax holidays for qualifying firms. Within two months of enactment, Seoul-based startups secured over KRW 600 billion (≈USD 450 million) in new funding, and the Ministry of Health announced plans for a national cell-processing center by 2026. These government-led incentives are accelerating domestic clinical pipelines and foreign partnership inflows.
Europe maintains a strong foothold thanks to coordinated EMA pathways, pan-EU research grants, and rising hospital adoption of CAR-T and gene therapies. Horizon Europe funding, valued at EUR 95 billion, underwrites multicentre trials, while cross-border manufacturing networks lower supply bottlenecks. Uptake is spearheaded by Germany, France, and the UK, whose public health systems are negotiating outcomes-based pricing to balance innovation with affordability. Continued focus on making advanced therapies cost-effective keeps Europe a vital, if more regulated, pillar of global growth.
Germany Regenerative Medicine Market
Germany leverages its engineering prowess and clinical depth. Frankfurt will host the 21st Global Summit on Stem Cell & Regenerative Medicine in June 2025, expected to draw 3,000 delegates and catalyze new academic-industry consortia. Concurrently, the Fraunhofer Institute launched a bioprinting centre in Leipzig focused on GMP-grade cardiac patches, funded by a EUR 40 million federal-state grant. These developments, plus Germany’s “Innovation Booster” tax credit effective January 2025, are attracting foreign biotechs to establish EU manufacturing bases, reinforcing the country’s status as Europe’s production hub for advanced therapies.
United Kingdom Regenerative Medicine Market
Post-Brexit regulatory agility is paying dividends for the UK. On January 31 2025, NICE approved the first gene therapy for sickle-cell disease for routine NHS use, signaling willingness to reimburse high-value curatives under managed-access agreements. Simultaneously, the Cell and Gene Therapy Catapult expanded its Stevenage manufacturing innovation centre, adding 6,000 m² of GMP space co-funded by a GBP 60 million government-industry package. These moves reduce commercialization friction and encourage overseas developers to run early access programs in the UK, ensuring the country remains a leading European launchpad for regenerative medicines.
To know more about the market opportunities by region and country, click here to
Buy The Complete Report
Regenerative Medicine Market Drivers
Rising Burden of Chronic and Degenerative Diseases
Advancements in Stem Cell and Gene Editing Technologies
High Cost and Complex Manufacturing Processes
Regulatory and Ethical Challenges
Request Free Customization of this report to help us to meet your business objectives.
The regenerative medicine market is characterized by dynamic collaborations and technological advancements. A notable example is the strategic partnership between Cryoport and Minaris Regenerative Medicine, aiming to enhance the development of cell and gene therapies by streamlining production and logistics processes. In the UK, Heart Biotech is pioneering heart valve technology that integrates with the patient's own tissue, reducing the need for repeated surgeries, especially in children with congenital heart defects. These specific initiatives underscore the market's focus on innovative solutions and strategic alliances to accelerate the availability and effectiveness of regenerative therapies.
SkyQuest’s ABIRAW (Advanced Business Intelligence, Research & Analysis Wing) is our Business Information Services team that Collects, Collates, Correlates, and Analyses the Data collected using Primary Exploratory Research backed by robust Secondary Desk research.
SkyQuest’s study suggests that The regenerative medicine market is primarily driven by the rising prevalence of chronic and degenerative diseases, which creates strong demand for curative therapies that restore damaged tissues and organs. North America dominates the market, supported by robust funding, advanced regulatory frameworks, and extensive clinical trial activity. The cell therapy segment leads due to its broad applications and growing FDA approvals. However, high manufacturing costs and complex production processes remain significant restraints, limiting accessibility and scalability. Additionally, advancements in gene editing and stem cell technologies serve as a key secondary driver, enabling personalized treatments and accelerating innovation. Together, these factors shape the dynamic growth and challenges within the regenerative medicine landscape.
| Report Metric | Details |
|---|---|
| Market size value in 2024 | USD 43.90 Billion |
| Market size value in 2033 | USD 166.95 Billion |
| Growth Rate | 16% |
| Base year | 2024 |
| Forecast period | 16% |
| Forecast Unit (Value) | USD Billion |
| Segments covered |
|
| Regions covered | North America (US, Canada), Europe (Germany, France, United Kingdom, Italy, Spain, Rest of Europe), Asia Pacific (China, India, Japan, Rest of Asia-Pacific), Latin America (Brazil, Rest of Latin America), Middle East & Africa (South Africa, GCC Countries, Rest of MEA) |
| Companies covered |
|
| Customization scope | Free report customization with purchase. Customization includes:-
|
To get a free trial access to our platform which is a one stop solution for all your data requirements for quicker decision making. This platform allows you to compare markets, competitors who are prominent in the market, and mega trends that are influencing the dynamics in the market. Also, get access to detailed SkyQuest exclusive matrix.
Table Of Content
Executive Summary
Market overview
Parent Market Analysis
Market overview
Market size
KEY MARKET INSIGHTS
COVID IMPACT
MARKET DYNAMICS & OUTLOOK
Market Size by Region
KEY COMPANY PROFILES
Methodology
For the Regenerative Medicine Market, our research methodology involved a mixture of primary and secondary data sources. Key steps involved in the research process are listed below:
1. Information Procurement: This stage involved the procurement of Market data or related information via primary and secondary sources. The various secondary sources used included various company websites, annual reports, trade databases, and paid databases such as Hoover's, Bloomberg Business, Factiva, and Avention. Our team did 45 primary interactions Globally which included several stakeholders such as manufacturers, customers, key opinion leaders, etc. Overall, information procurement was one of the most extensive stages in our research process.
2. Information Analysis: This step involved triangulation of data through bottom-up and top-down approaches to estimate and validate the total size and future estimate of the Regenerative Medicine Market.
3. Report Formulation: The final step entailed the placement of data points in appropriate Market spaces in an attempt to deduce viable conclusions.
4. Validation & Publishing: Validation is the most important step in the process. Validation & re-validation via an intricately designed process helped us finalize data points to be used for final calculations. The final Market estimates and forecasts were then aligned and sent to our panel of industry experts for validation of data. Once the validation was done the report was sent to our Quality Assurance team to ensure adherence to style guides, consistency & design.
Analyst Support
Customization Options
With the given market data, our dedicated team of analysts can offer you the following customization options are available for the Regenerative Medicine Market:
Product Analysis: Product matrix, which offers a detailed comparison of the product portfolio of companies.
Regional Analysis: Further analysis of the Regenerative Medicine Market for additional countries.
Competitive Analysis: Detailed analysis and profiling of additional Market players & comparative analysis of competitive products.
Go to Market Strategy: Find the high-growth channels to invest your marketing efforts and increase your customer base.
Innovation Mapping: Identify racial solutions and innovation, connected to deep ecosystems of innovators, start-ups, academics, and strategic partners.
Category Intelligence: Customized intelligence that is relevant to their supply Markets will enable them to make smarter sourcing decisions and improve their category management.
Public Company Transcript Analysis: To improve the investment performance by generating new alpha and making better-informed decisions.
Social Media Listening: To analyze the conversations and trends happening not just around your brand, but around your industry as a whole, and use those insights to make better Marketing decisions.
REQUEST FOR SAMPLE
The regenerative agriculture market is expected to post a CAGR of 14.6% from 2025 to 2032 due to increasing consumer demand for sustainable and environmentally-friendly practices, paired with governmental support towards eco-friendly sustainable agricultural practices.
The major product segments in the regenerative agriculture market are crop-based and livestock-based products. Crop-based products capture most of the market share primarily due to their role in food and livestock production; whereas livestock based products are also creating their own market share, related to sustainable meat and dairy.
Currently crop-based products have the largest market share in the regenerative agriculture market, because they are the only source of food and satisfy consumer desires for sustainable and organic locally sourced produce.
Increased demand for food throughout Asia Pacific is contributing the most to the growth of regenerative agriculture among food producers and food distributors. There is increased consumer awareness related to sustainability. Growth is also influenced by government initiatives, such as China's Soil Ten Plan and India’s Rashtriya Krishi Vikas Yojana, which promote sustainable agriculture initiatives.
Precision agriculture technologies will drive change in regenerative farming by improving sustainability and transparency through things like blockchain traceability and data monitoring that optimize usage of resources and improve soil health.
Future opportunities in regenerative agriculture will be in microbial inoculants, biostimulants, and enhancing consulting services for improving soil health, increased crop yield, and transition to sustainability.
Recent innovations include Nestlé's NESCAFÉ Plan 2030 that is funding regenerative practices, and General Mills' collaboration with Regrow Agriculture to track sustainability across 175 million acres using precision tools and technology and analytics.
Emerging trends are including AI enabled precision agriculture, increased use of organic food, and sustainable sustainable partnerships with growing emphasis on data and eco-friendly practices by 2032.
Want to customize this report? This report can be personalized according to your needs. Our analysts and industry experts will work directly with you to understand your requirements and provide you with customized data in a short amount of time. We offer $1000 worth of FREE customization at the time of purchase.
Feedback From Our Clients