
Report ID: SQMIG35A2534

Report ID: SQMIG35A2534
sales@skyquestt.com
USA +1 351-333-4748

Report ID:
SQMIG35A2534 |
Region:
Global |
Published Date: December, 2025
Pages:
187
|Tables:
129
|Figures:
77



Global Medical Device Contract Manufacturing Market size was valued at USD 70.83 Billion in 2024 and is poised to grow from USD 78.9 Billion in 2025 to USD 187.15 Billion by 2033, growing at a CAGR of 11.4% during the forecast period (2026–2033).
The medical device contract manufacturing market for medical devices is experiencing robust growth, driven by increased demand for the medical devices and technological advancements in the market has shown resilience and recovery, with a significant increase in the outsourcing of medical devices. The increase is driven by higher demand for devices such as ventilators and IVD-related products, putting companies in touch with contractors for their expertise and capabilities. The medical device contract manufacturing market with its diverse range of devices and product lines drives the demand for contract manufacturing services.
Companies that do not have in-house manufacturing facilities are turning to more contract manufacturers, fueling market growth. The initiatives taken to widen the acceptance and usage of medical devices across the globe, such as new regulatory framework and strategic approach also contribute to the market expansion. The in vitro diagnostic (IVD) device segment is poised to hold a significant share of the market demand for providing point-of-care devices is increasing. The market is driven by accessibility and expansion by key market players in North America, especially the US, as a key contributor to the market.
How is Additive manufacturing (3D printing) Technology bringing Transformation in the Market?
Additive manufacturing (3D printing) technology is transforming the market dynamics. This technology allows for faster and cost-effective production of devices having complex geometries. They also make the design more flexible, quicken prototyping process, and reduce time-to-market. All of these are critical factors for OEMs and healthcare providers. University Hospital Basel, for example, use of 3D Systems’ EXT 220 MED printer to manufacture a customized, MDR-compliant PEEK facial implant in March 2025. This underlines the technology’s potential to bring about transformation in the market globally.
To get more insights on this market click here to Request a Free Sample Report
The global medical device contract manufacturing market is segmented into product class, service type, therapeutic area, end user, and region. By product class, the market is classified into Class I, Class II, and Class III. Depending on service type, it is divided into device development and manufacturing, quality management, packaging and assembly services, and others. According to therapeutic area, the market is categorized into cardiovascular devices, orthopedic devices, ophthalmic devices, diagnostic devices, respiratory devices, surgical instruments, dental, and others. By end user, the market is classified into Original Equipment Manufacturers (OEMs), pharmaceutical & biopharmaceutical companies, and others. Regionally, the medical device contract manufacturing market is analyzed across North America, Europe, Asia-Pacific, Middle East & Africa, and Latin America.
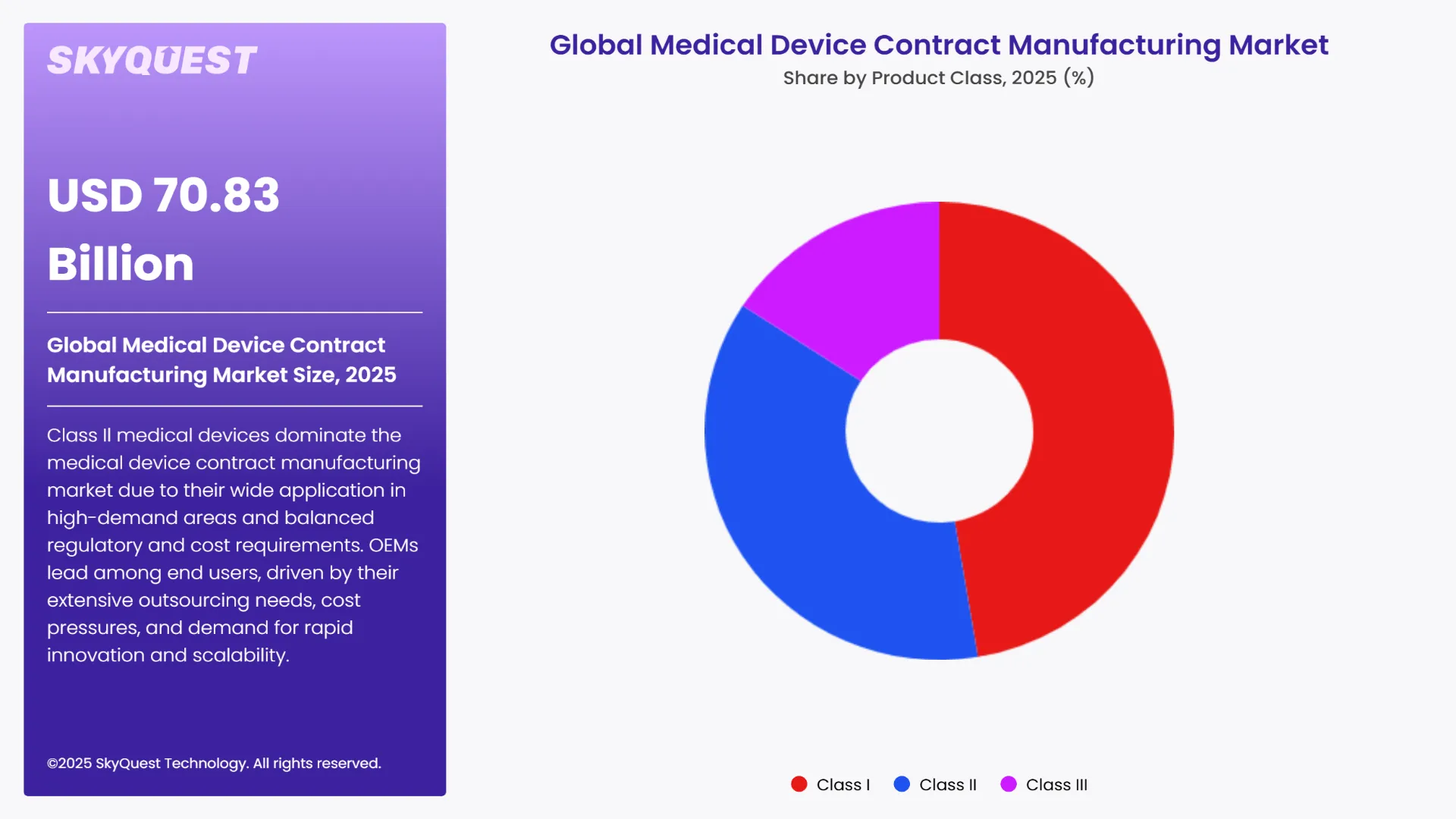
As per the 2024 global medical device contract manufacturing market analysis, the Class II medical devices sub segment led the market by holding the largest share. This is due to the fact that they find applications across various high-demand therapeutic areas like diagnostic imaging, surgical instruments, and infusion pumps. This category includes catheters, syringes, surgical gloves, blood pressure monitors, pregnancy test kits, spectacles, and blood samples. These devices require more complex design, regulatory compliance, and precision manufacturing than Class I. At the same time, they require less capital expense than Class III. This balanced position is making them ideal for outsourcing and the most dominant class in the market.
The Class I sub segment is anticipated to grow at the highest CAGR during the forecast period of 2025-2032. This high growth rate can be as a result of the rising demand for advanced and life-saving technologies such as implantables, pacemakers, and neurostimulators. They require elaborate methods of engineering, strict compliance with regulatory codes, and sterile manufacturing environments. OEMs, therefore, prefer to rely upon contract manufacturers to manage complexity and reduce time-to-market. As a result, the category is growing at a steady pace.
The OEMs category in the end user segment is the most dominant in the global medical device contract manufacturing market. This is because they have significant outsourcing needs for the design, prototyping, production, and regulatory compliance of their products. The pressure to reduce costs is increasing consistently. These end users also require faster time-to-market and focus on innovation. That is why OEMs depend heavily on contract manufacturers. Their consistent demand and long-term partnerships with the manufacturers make them the largest revenue contributors in the market.
During the forecast period, the Pharmaceutical and biopharmaceutical companies’ sub-segment is expected to grow with a notable CAGR. The major driving force of this increase is the convergence of drugs and devices. Many biotechnology and pharmaceutical companies such as Bristol-Myers Squibb (BMS), GlaxoSmithKline, Novartis, Eli Lilly & Co., AstraZeneca invest in synthetic devices. Combination products like auto-injectors and drug-delivery systems are seeing growth and this is accelerating demand for device manufacturing expertise.
To get detailed segments analysis, Request a Free Sample Report
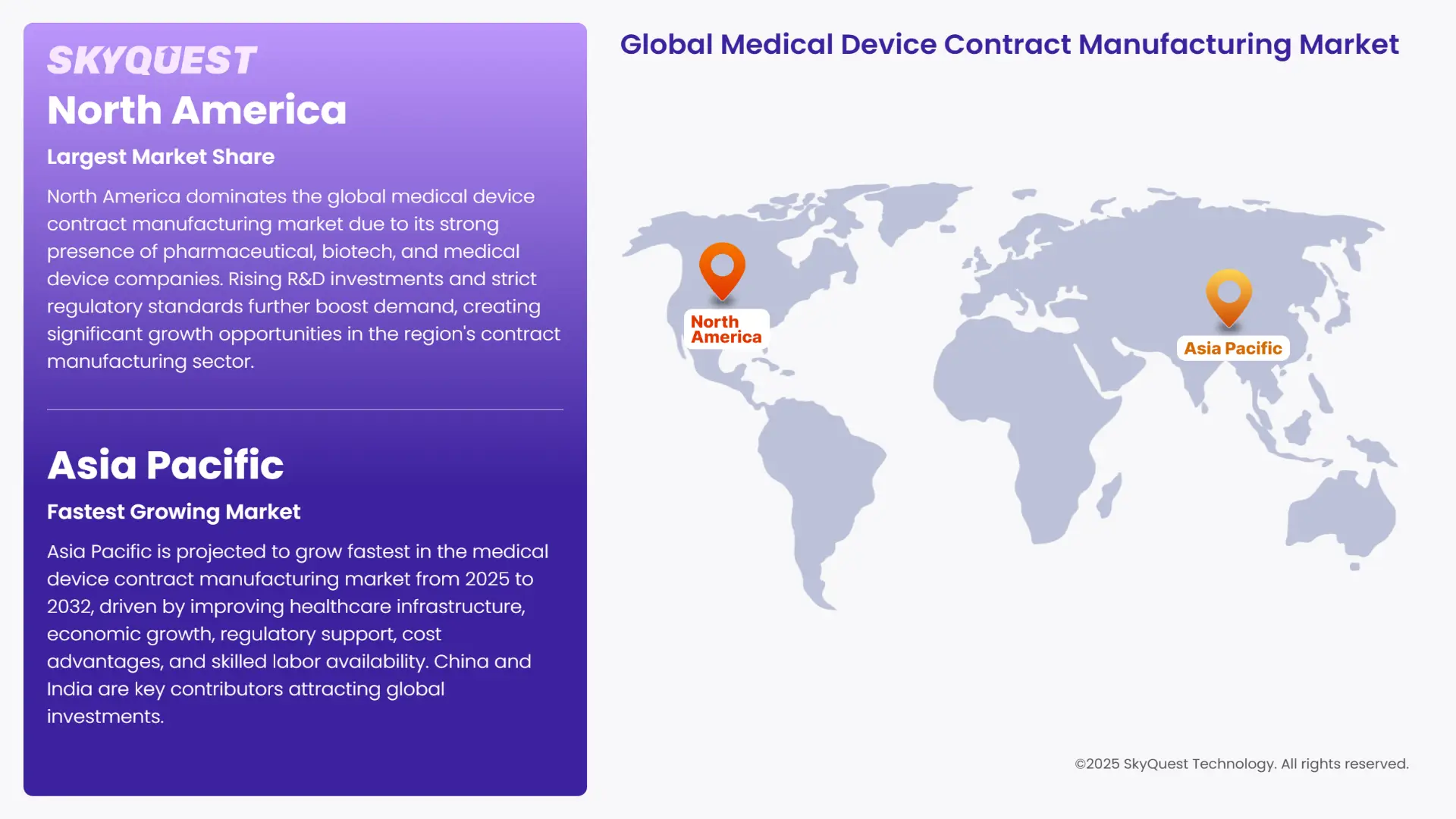
The North America medical device contract manufacturing market share emerges as the most dominant market in the world. The region's importance can be attributed to the many established biotechnology, pharmaceutical and medical device companies in the region. In addition, increasing R&D investments by life sciences and pharmaceutical companies are expected to increase the sector's demand for contract manufacturing independently. Strict regulation manufacturing and quality issues are also expected to provide opportunities for growth in medical device contract manufacturing.
U.S. Medical Device Contract Manufacturing Market
In North America, U.S. leads the medical device contract manufacturing market share especially due to its strong R&D investments, strict regulatory framework, and high demand for advanced and modern healthcare solutions. Many key players of the market such as Jabil, Flex Ltd., Plexus Corp. and Sanmina Corporation are headquartered here. The presence of major OEMs and contract manufacturers, along with favorable reimbursement policies and innovation in digital health and minimally invasive technologies, continue to help in its market growth. In June 2023, Arterex, a medical device contract manufacturer, acquired NextPhase Medical Devices LLC, a class II and III medical device contract manufacturer. It was done to double Arterex’s size and create a global contract manufacturing platform which would garner 70% of revenues from the U.S. market.
Canada Medical Device Contract Manufacturing Market
Canada in North America is just behind the U.S. The country’s medical device contract manufacturing market growth is due to its strong public healthcare infrastructure, skilled workforce, and regulatory pathways which are quite streamlined. Growth is particularly strong in orthopedic, diagnostic, and home healthcare devices. Strategic investments in health technology innovation and government incentives are also helping in promoting local production and attracting global partnerships. In May 2023, Cybeats signed a license agreement with StarFish Medical which is Canada's largest contract medical device manufacturer. This agreement was to provide its SBOM Studio platform for managing software supply chain cybersecurity.
Asia Pacific is predicted to grow at the highest rate in the medical device contract manufacturing market growth over the projection period of 2025-2032. The growth in the region is led by the improving healthcare infrastructure and economic growth in the developing countries of this region. This can be attributed to supportive regulatory changes, especially in countries like India, and cost-saving opportunities in Asian countries. Moreover, cGMP-compliant areas are expected to be established market players in the region will boost the market. Moreover, China is a major producer of low-cost electronics and goods. Therefore, it is expected to attract a significant number of investors. Furthermore, labour flexibility is greater in Asia Pacific due to lower production costs, tax advantages and availability of relatively cheap skilled labour.
China Medical Device Contract Manufacturing Market
The China medical device contract manufacturing market expansion can be credited to various reasons. This expansion can be contributed to a changing market landscape that is seeing an expansive manufacturing base, low production costs, and rising domestic demand. The country’s supporting government initiatives also helps in medtech innovation. China revised its Medical Device Regulation in January 2025. This strengthens lifecycle oversight, streamlines approvals, and supports innovation. It provides contract manufacturing clarity, quality management, and market access. China's export capacity and partnerships with global OEMs also accounts for the country being a primary hub for medical device production.
India Medical Device Contract Manufacturing Market
India’s market growth is spurred by its supporting government schemes like the “Make in India” scheme. The country’s medical device contract manufacturing market is quite cost-effective. It has a skilled workforce, and an expanding infrastructure strengthened by increasing healthcare investments. Its focus on precision engineering and growth in domestic OEMs are also huge factors prompting its rise as an emerging destination for contract manufacturing, especially in those devices related to diagnostics, consumables, and low-to-mid-risk devices.
Europe held a significant medical device contract manufacturing market share in 2024 and is likely to continue so during the forecast period of 2025-2032. The region has a mature and technologically advanced market. Regulations are strictly abided by demand for complex medical technologies is high and OEMs are present in abundance in the region. The Medical Device Regulation (MDR) puts heavy emphasis on quality, innovation, and compliance.
Germany Medical Device Contract Manufacturing Market
Germany has the largest European medical device contract manufacturing market growth. Germany has a strong manufacturing infrastructure and a world-class R&D ecosystem. Germany houses many OEMs and specialized CMOs in diagnostics, surgical instruments and orthopedic devices. Germany’s dedication to comply with EU MDR promotes demand in the country. Strategic investments in digital health and Industry 4.0 also play important roles to enhance Germany’s role as a key hub in the European market.
France Medical Device Contract Manufacturing Market
The France medical device contract manufacturing market is also experiencing steady growth. It is supported by its growing ecosystem of medtech industry. France also hosts many OEMs and contract manufacturers working in diagnostics, surgical devices, and implants. The nation’s companies also observe strong presence in R&D and public-private partnerships, this makes France a key player in Europe's medical device contract manufacturing landscape.
To know more about the market opportunities by region and country, click here to
Buy The Complete Report
Growing Demand for Medical Devices
Increasing Outsourcing in the Healthcare Sector
Regulatory Compliance and Quality Control Challenges
Supply Chain Disruptions
Request Free Customization of this report to help us to meet your business objectives.
The competitive landscape of the medical device contract manufacturing market is composed of a wide variety of players including established companies, specialty contractors and emerging companies. These companies compete based on their technological expertise, manufacturing capabilities, regulatory compliance, and ability to provide end-to-end services, from product design to production and post-market support. Other tactics that companies in the market follow to stay at the top of their game include strategic partnerships, mergers and acquisitions, and investment in newer technologies.
Technological Advances in Manufacturing
Increased Focus on Sustainability and Environmentally Friendly Practices
SkyQuest’s ABIRAW (Advanced Business Intelligence, Research & Analysis Wing) is our Business Information Services team that Collects, Collates, Correlates, and Analyses the Data collected using Primary Exploratory Research backed by robust Secondary Desk research.
According to SkyQuest analysis, contract manufacturing of medical devices is experiencing strong growth, driven by increasing demand for medical devices, increasing exports OEMs are relying on contract manufacturers to consume production and meet the requirements and focus on other areas. However, regulatory challenges and supply chain disruptions are severely hampering the growth of the market. Technological advances and sustainability efforts represent key trends shaping the future of the industry. As contract manufacturers continue to innovate and adopt more flexible business models, they are well positioned to play a key role in global healthcare.
| Report Metric | Details |
|---|---|
| Market size value in 2024 | USD 70.83 Billion |
| Market size value in 2033 | USD 187.15 Billion |
| Growth Rate | 11.4% |
| Base year | 2024 |
| Forecast period | 2026–2033 |
| Forecast Unit (Value) | USD Billion |
| Segments covered |
|
| Regions covered | North America (US, Canada), Europe (Germany, France, United Kingdom, Italy, Spain, Rest of Europe), Asia Pacific (China, India, Japan, Rest of Asia-Pacific), Latin America (Brazil, Rest of Latin America), Middle East & Africa (South Africa, GCC Countries, Rest of MEA) |
| Companies covered |
|
| Customization scope | Free report customization with purchase. Customization includes:-
|
To get a free trial access to our platform which is a one stop solution for all your data requirements for quicker decision making. This platform allows you to compare markets, competitors who are prominent in the market, and mega trends that are influencing the dynamics in the market. Also, get access to detailed SkyQuest exclusive matrix.
Table Of Content
Executive Summary
Market overview
Parent Market Analysis
Market overview
Market size
KEY MARKET INSIGHTS
COVID IMPACT
MARKET DYNAMICS & OUTLOOK
Market Size by Region
KEY COMPANY PROFILES
Methodology
For the Medical Device Contract Manufacturing Market, our research methodology involved a mixture of primary and secondary data sources. Key steps involved in the research process are listed below:
1. Information Procurement: This stage involved the procurement of Market data or related information via primary and secondary sources. The various secondary sources used included various company websites, annual reports, trade databases, and paid databases such as Hoover's, Bloomberg Business, Factiva, and Avention. Our team did 45 primary interactions Globally which included several stakeholders such as manufacturers, customers, key opinion leaders, etc. Overall, information procurement was one of the most extensive stages in our research process.
2. Information Analysis: This step involved triangulation of data through bottom-up and top-down approaches to estimate and validate the total size and future estimate of the Medical Device Contract Manufacturing Market.
3. Report Formulation: The final step entailed the placement of data points in appropriate Market spaces in an attempt to deduce viable conclusions.
4. Validation & Publishing: Validation is the most important step in the process. Validation & re-validation via an intricately designed process helped us finalize data points to be used for final calculations. The final Market estimates and forecasts were then aligned and sent to our panel of industry experts for validation of data. Once the validation was done the report was sent to our Quality Assurance team to ensure adherence to style guides, consistency & design.
Analyst Support
Customization Options
With the given market data, our dedicated team of analysts can offer you the following customization options are available for the Medical Device Contract Manufacturing Market:
Product Analysis: Product matrix, which offers a detailed comparison of the product portfolio of companies.
Regional Analysis: Further analysis of the Medical Device Contract Manufacturing Market for additional countries.
Competitive Analysis: Detailed analysis and profiling of additional Market players & comparative analysis of competitive products.
Go to Market Strategy: Find the high-growth channels to invest your marketing efforts and increase your customer base.
Innovation Mapping: Identify racial solutions and innovation, connected to deep ecosystems of innovators, start-ups, academics, and strategic partners.
Category Intelligence: Customized intelligence that is relevant to their supply Markets will enable them to make smarter sourcing decisions and improve their category management.
Public Company Transcript Analysis: To improve the investment performance by generating new alpha and making better-informed decisions.
Social Media Listening: To analyze the conversations and trends happening not just around your brand, but around your industry as a whole, and use those insights to make better Marketing decisions.
REQUEST FOR SAMPLE
Class II (moderate complexity) devices, such as surgical gloves, diagnostic imaging, and infusion pumps are most commonly contracted out to contract manufacturers because they cover moderate levels of regulatory complexity and are applied in a wide variety of high-demand therapeutic areas.
The main drivers include the increased demand for medical devices, more OEMs outsourcing to reduce costs, searching for innovation, and the introduction of technology such as 3D Printing and automation.
Regulatory compliance creates challenges due to differences in worldwide standards. Manufacturers must adhere to both the FDA, EMA, and a multitude of other regulatory bodies, therefore it is essential to find partners who have experience in and understand compliance.
Although the economy has slowed, the medical device contract manufacturing market has managed to withstand the economic fluctuations. Increased outsourcing from OEMs and consistent demand for medical devices (including ventilators and high-demand IVD products) led to a significant share of the medical device market being added even during a recession in years like 2020.
The challenges facing the industry are of major concern given the complex regulatory requirements, which are coupled with supply chain disruptions globally, making production difficult, costly, and challenging for new devices to enter the market.
Global Medical Device Contract Manufacturing Market size was valued at USD 70.83 Billion in 2024 and is poised to grow from USD 78.9 Billion in 2025 to USD 187.15 Billion by 2033, growing at a CAGR of 11.4% during the forecast period (2026–2033).
Thermo Fisher Scientific Inc. (United States), Jabil Inc. (United States), Flex Ltd. (United States), Plexus Corp. (United States), Sanmina Corporation (United States), Integer Holdings Corporation (United States), TE Connectivity Ltd. (Switzerland), Nipro Corporation (Japan), Onex Corporation (Celestica Inc.) (Canada), West Pharmaceutical Services, Inc. (United States), Benchmark Electronics Inc. (United States), EQT Group (Recipharm AB) (Sweden), Gerresheimer AG (Germany), Kimball Electronics, Inc. (United States), Nortech Systems Incorporated (United States), Carclo plc (United Kingdom), Nolato GW, Inc. (Sweden), Viant Medical Holdings, Inc. (United States), Tecomet, Inc. (United States), SMC Corporation (United States)
The key driver of the medical device contract manufacturing market is the increasing demand for cost-efficient production, allowing healthcare companies to outsource manufacturing, focus on innovation, reduce time-to-market, and meet the growing global demand for advanced medical devices.
A key market trend in the medical device contract manufacturing market is the adoption of advanced manufacturing technologies like automation, additive manufacturing (3D printing), and digitalization to improve precision, reduce costs, and accelerate product development cycles.
North America accounted for the largest share in the medical device contract manufacturing market, driven by a well-established healthcare infrastructure, high demand for innovative medical devices, strong regulatory support, and the presence of major medical device manufacturers and contract service providers.
Want to customize this report? This report can be personalized according to your needs. Our analysts and industry experts will work directly with you to understand your requirements and provide you with customized data in a short amount of time. We offer $1000 worth of FREE customization at the time of purchase.
Feedback From Our Clients