
Report ID: SQMIG35A3049

Report ID: SQMIG35A3049
sales@skyquestt.com
USA +1 351-333-4748

Report ID:
SQMIG35A3049 |
Region:
Global |
Published Date: June, 2025
Pages:
192
|Tables:
189
|Figures:
79



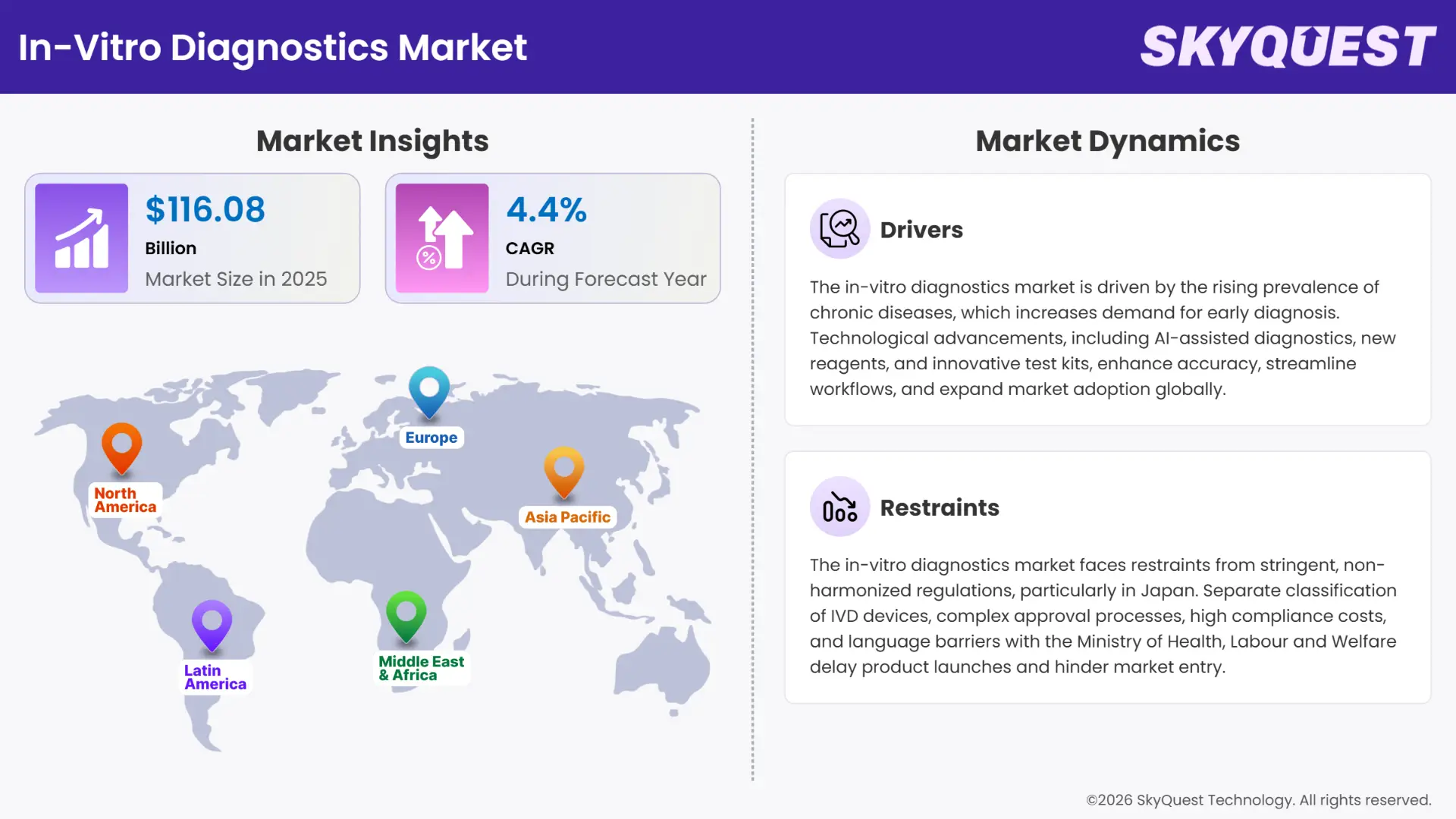
Global In-Vitro Diagnostics Market size was valued at USD 111.19 Billion in 2024 and is poised to grow from USD 116.08 Billion in 2025 to USD 163.82 Billion by 2033, growing at a CAGR of 4.4% during the forecast period (2026–2033).
The rise in prevalence of chronic and infectious disease, advancement in technologies and increase in healthcare awareness are the major factor for the growth of the in-vitro diagnostics market. The increasing prevalence of infectious and chronic diseases, advancement in diagnostic technologies, and rise in healthcare awareness are driving the growth of the in-vitro diagnostics market. According to SkyQuest Analysis, it was reported that around 70% of clinical decisions are influenced by IVD testing, highlighting its critical role in modern healthcare facility. This rise in diagnostic testing fuels the demand for IVD products and propels overall market expansion.
Further, the growing geriatric population, surge in adoption of personalized medicine, and increase in government initiatives to increase diagnostic infrastructure further contribute to growth of the market. In addition, the rise in burden of disease such as cancer, diabetes and cardiovascular disease boost the demand for early and accurate diagnostic solutions.
The advancement in technology in IVD devices such as molecular diagnostics, point-of-care testing kits and AI-integrated diagnostic tools are further driving the in-vitro diagnostics market growth. The widespread availability of wearable diagnostic devices and self-testing kits further accelerate the demand mostly in underserved and remote regions. Furthermore, the increasing popularity of at-home diagnostic and rise in testing frequency due to emerging health threats such as COVID-19 pandemic have surge the consumer base which increase the demand across all age groups.
Further, it was also analysed that nearly 75% of hospitals globally have integrated some form of molecular IVD technology, indicating broad institutional acceptance. Diagnostic laboratories are capturing a higher share of the market by leveraging automation, cloud-based data analytics, and customized test panels, directly competing with traditional hospital-based testing services. Furthermore, increased investment in research and development activities by manufacturers is fueling the introduction of highly sensitive, rapid, and cost-effective diagnostic tools, accelerating innovation and product diversification in the market. Roche Diagnostics’ launch of the cobas® pulse system, a smart POC device for professional use, reflects the impact of increased R&D investment on innovation within the IVD industry.
However, challenges such as regulatory complexities, price pressure due to reimbursement issues, and the need for skilled professionals may restrain market growth to some extent.
How is AI Advancing in In-Vitro Diagnostics industry?
The in-vitro diagnostics industry is rapidly evolving with the integration of advanced technologies such as artificial intelligence and machine learning. These technology process huge amount of data including imaging, patient records and test results with precision and remarkable speed. This allow IVD tools in order to provide more accurate diagnostic by detecting the anomalies and patterns which might be missed by human observation. AI-driven IVD platform aims to analyze biomarkers more effectively which lead to earlier detection of disorder such as sepsis, genetic disorder or cancer.
AI and machine learning algorithms can process vast amounts of data, including patient records, imaging, and test results, with remarkable speed and precision. This allows IVD tools to provide more accurate diagnostics by detecting patterns and anomalies that might be missed by human observation.AI-driven IVD platforms can analyze biomarkers more effectively, leading to earlier detection of diseases like cancer, sepsis, or genetic disorders.
AI-powered point-of-care testing devices are increasing used for real-time diagnostic at the bedside or in remote location. These tools provide rapid results to enhance patient care in critical situation. AI-based POCT devices helps to analyze blood glucose levels instantly. It also acts as a cardiac biomarker or infection, which enable immediate treatment decision in emergency sitting.
Furthermore, integrating AI in IVD tools helps to reduce the cost which is associated with diagnostic errors, lengthy manual procedures and hospital readmissions. Thus, AI enhance accuracy, efficiency along with lowering the healthcare costs.
To get more insights on this market click here to Request a Free Sample Report
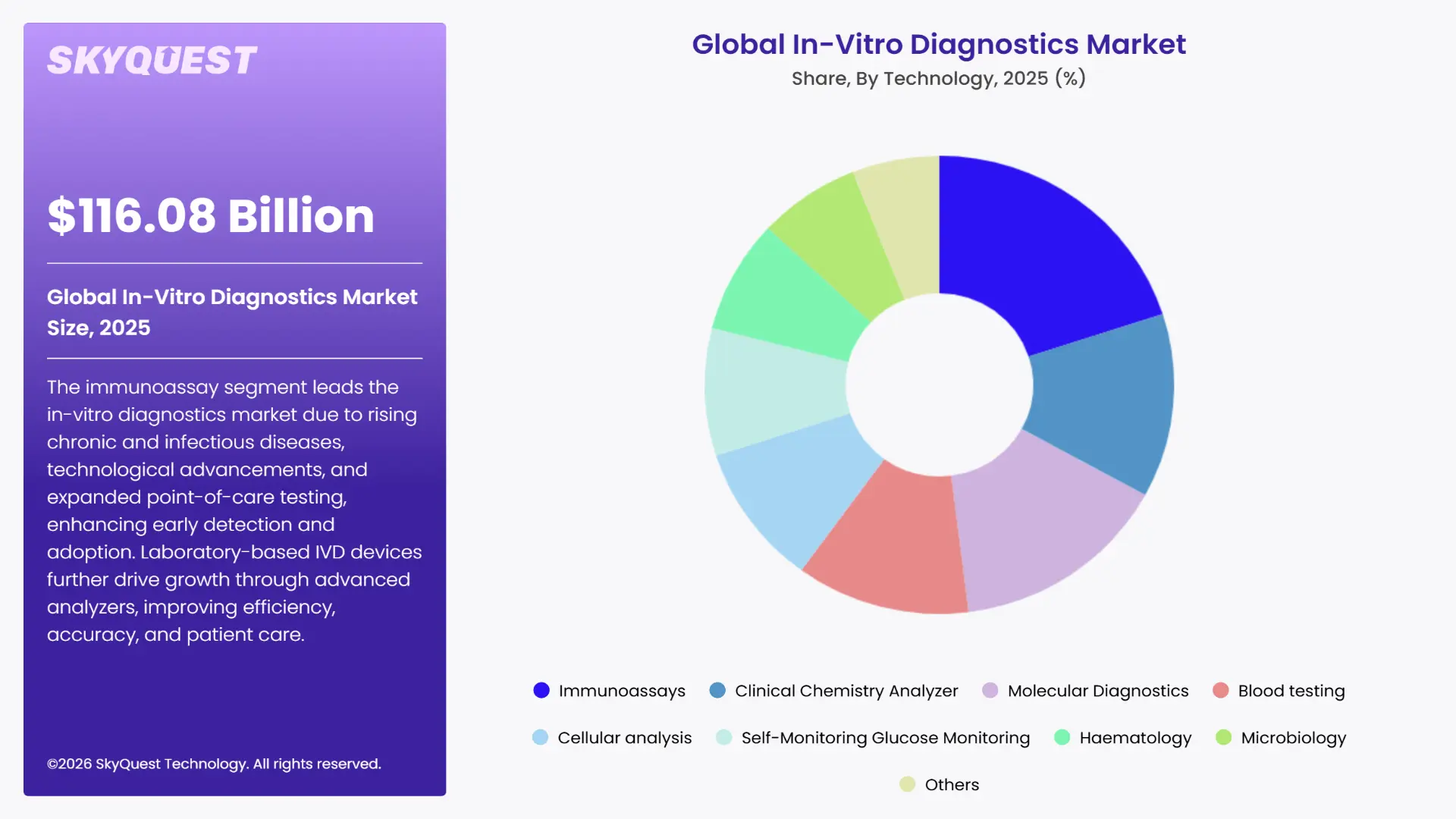
The global in-vitro diagnostics market is segmented by Product, By Technology, By Specimen, By Site of Testing, By Application, By End User and region. Based on By Product, the market is segmented into Instrument & Reagent, Software and Services. Based on By Technology, the market is segmented into Immunoassays, Clinical Chemistry Analyzer, Molecular Diagnostics, Blood testing, Cellular analysis, Self-Monitoring Glucose Monitoring, Hematology, Microbiology and Others. Based on By Specimen, the market is segmented into Blood, CSF, Urine, Abscess, Stool, Body Fluid and Other Specimens. Based on By Site of Testing, the market is segmented into Laboratory Tests and Point-of-Care Tests. Based on By Application, the market is segmented into Infectious Diseases, Diabetics, Cardiology, Oncology, Nephrology, Autoimmune Disease, Drug Testing and Others. Based on By End User, the market is segmented into Hospitals & Clinics, Clinical Laboratories, Blood Banks, Home Care Settings, Pharmaceutical & Biotechnology Companies, Academic Institutes and Other End Users. Based on region, the in-vitro diagnostics market is segmented into North America, Europe, Asia Pacific, Latin America and Middle East & Africa.
How is the Immunoassay Segment Driving Market Leadership?
The immunoassay segment dominates the in-vitro diagnostics market due to rising prevalence of chronic and infectious disease, advancement in technology and expansion of point of care testing. In April 2024, the World Health Organization has expanded the Prequalification (PQ) scope for IVDs, which cover HbA1c point-of-care tests and devices in order to measure blood glucose in capillary blood. These helps to enhance early disease surveillance and detection, thereby amplifying the adoption of immunoassay technologies.
Further, the Haematology segment emerged as the fastest-growing market during the forecast period. A key driver of this growth is the Abbott ARCHITECT SARS-CoV-2 IgG assay, approved by the U.S. FDA, which detects IgG antibodies against COVID-19 with 100% sensitivity and 99% specificity. Its extensive adoption in hospitals and laboratories for accurate antibody detection underscores the reliability of immunoassays, thereby accelerating their usage and fueling the overall expansion of the IVD market.
How is laboratory based IVD-devices driving the growth of the In-Vitro Diagnostics market?
Based on the 2024 global in-vitro diagnostics market forecast, the laboratory-based segment dominates the market due to advancement in technology. In 2024, Beckman Coulter Diagnostics, one of the clinical diagnostic companies, announced the launch of DxC 500i Clinical Analyzer. It is a clinical chemistry and immunoassay analyzer. The healthcare system adopts the network laboratory operational model for better efficiency and patient access.
Sensa Core, one of the leading manufacturers of medical diagnostic solutions, announced the launch of ST 200 CS and ST 200 SC blood gas analyzer. These analyzers represent a significant advancement in blood gas analysis technology which offer healthcare professionals the tools which they need in order to offer possible care for patient. Thus, this technological advancement significantly drives the growth in laboratory based IVD market.
To get detailed segments analysis, Request a Free Sample Report
The Asia-Pacific in-vitro diagnostics market share is driven by a geriatric population, increase in prevalence of chronic diseases and rise in demand for early diagnostics. Government initiatives across the region promote healthcare infrastructure and accessibility. Expansion of local operations by major players improves market reach, customer support, and availability of advanced diagnostic solutions.
Japan In-Vitro Diagnostics Market
The increasing geriatric population in Japan, rise in government initiative and surge in demand for early diagnostic drive the Japan in-vitro diagnostics market growth. According to our analysis, nearly 78% of individuals aged 75 and over have two or more co-occurring chronic conditions, and around 61% have three or more. Additionally, more than 85% of those aged 75 and older have at least one chronic disease. This surges the need for early and accurate diagnosis and drive the growth of IVD market
India In-Vitro Diagnostics Market
The rise in government initiative and surge in business expansion drive the growth of the in-vitro diagnostics market. In 2024, Sysmex strengthening its direct operations in India acts as a driver by improving market access and customer support for advanced IVD solutions. This expansion enhances the availability of a comprehensive range of clinical laboratory testing products, including hematology and clinical chemistry, boosting diagnostic capabilities.
Local presence allows faster service, better training, and tailored solutions to meet regional needs. As a result, Sysmex’s move accelerates market growth, encourages competition, and fosters innovation in India’s IVD sector, benefiting healthcare outcomes.
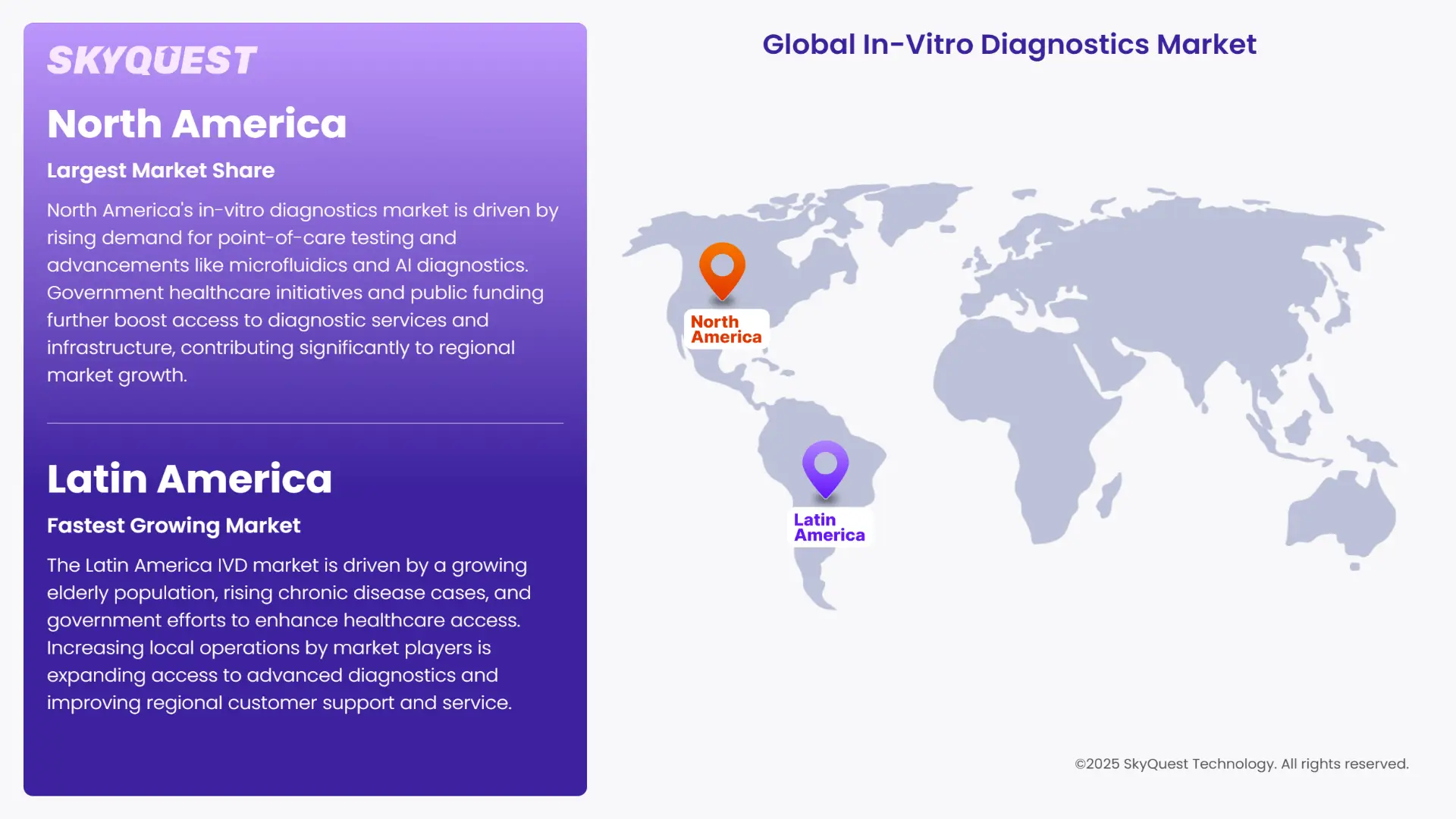
The North America in-vitro diagnostics market growth is propelled by growing demand for point-of-care testing and continuous technological advancements such as microfluidics and AI-based diagnostics. Government healthcare initiatives and public funding support broad access to diagnostic services and infrastructure improvements.
U.S. In-Vitro Diagnostics Market
The U.S. in-vitro diagnostics market share is experiencing significant growth driven by rising demand for point of care device. In January 2025, bioMérieux acquired 100% ownership of SpinChip for USD 156 million, aiming to strengthen its point-of-care presence. The first product launch from this acquisition is expected in 2026.
Further, The World Health Organization (WHO) is pleased to announce the third edition of the Week of Quality for In Vitro Diagnostics (IVDs), a specialised virtual training organized by the Local Production and Assistance (LPA) Unit within the Division of Innovation and Emerging Technologies (IET), in June 2025. Thus, technological innovations, increased awareness, strategic acquisitions, and global quality-focused initiatives propel the growth of the market.
Canada In-Vitro Diagnostics Market
In July 2024, Quest Diagnostic announced the acquisition of Canada-based LifeLabs, boosting diagnostic testing capacity, accelerating innovation, increasing lab service demand thereby driving significant growth in the IVD market.
Further increase in demand for molecular diagnostic test and rise in government healthcare initiative further propel the market growth. Canada’s publicly funded healthcare system (Medicare) supports widespread access to diagnostic testing. Government initiatives aimed at improving early disease detection and management, along with funding for healthcare infrastructure upgrades, boost IVD adoption.
The Latin America IVD market has several drivers, including a growing geriatric population, increasing prevalence of chronic diseases and repeated government initiatives to improve healthcare infrastructure and access in the region, as well as an ongoing demand for early diagnostics. As market participants begin to steadily grow their local operations to broaden market access, improve customer support and access to advanced diagnostic solutions.
Brazil In-Vitro Diagnostics Market
An increasing geriatric population, rising government initiatives in Brazil, as well as the growing demand for early diagnostic services drive growth in the Brazilian IVD market. Evidence from our analysis indicates that approximately 78% of aged individuals 75 years-old or older are reported to have two or more chronic conditions concurrently stuck with them, while almost by 61% indicated that they have reported three or more chronic conditions. Over 85% of individuals aged 75 years or older have reported having at least one chronic disease. This dictates the need for a timely and reliable diagnosis of chronic conditions which drives growth in the IVD market.
Mexico In-Vitro Diagnostics Market
The government initiatives and increase in business engagements drive growth in Mexico IVD market as Sysmex expands their direct operations to Mexico in 2024 while maturing their commercial agreements in the region, if improves access to advanced IVD solutions for customers to feel confident in their advanced clinical diagnostic testing requests while expanding the Product Availability in the Mexico region. Sysmex now has improved access to the entire range of clinical laboratory testing of products with an enhanced complete range of clinical laboratory testing product including hematology testing and a portion of clinical chemistries, enhancing diagnostic capabilities. As a result, Sysmex’s move accelerates market growth, encourages competition, and fosters innovation in India’s IVD sector, benefiting healthcare outcomes.
The Europe in-vitro diagnostics market trends are driven by innovation in non-invasive and user-friendly diagnostic solutions, increasing consumer acceptance and expanding self-testing product demand. Advances in personalized and home-based testing technologies further propel market growth. Manufacturing capacity expansions and strategic partnerships enhance production capabilities and support cost-effective scaling of molecular diagnostics.
Germany In-Vitro Diagnostics Market
The extension of Abingdon Health’s distribution agreement with Salignostics to launch Salistick™, the first saliva-based pregnancy test, across Germany and other European countries acts as a significant driver for the IVD market by introducing innovative, user-friendly, and non-invasive diagnostic solutions. This enhances consumer accessibility and acceptance of rapid testing, broadens the market for self-testing IVD products, and reflects growing demand for novel diagnostics. Such advancements support market expansion and technological progress in personalized and home-based IVD solutions across Europe.
UK In-Vitro Diagnostics Market
The UK IVD market growth is driven by manufacturing expansions and strategic partnerships. In August 2023, Sherlock Biosciences inaugurated a 36,000 square foot biomanufacturing facility in the UK, aiming to expand its diagnostic capacity and scale low-cost manufacturing of molecular diagnostics products.
In June 2023, Newfoundland Diagnostics entered into an exclusive supply agreement with Atomo Diagnostics for the distribution of rapid self-test HIV kits at retailers in the UK and Europe.
To know more about the market opportunities by region and country, click here to
Buy The Complete Report
Increase in prevalence of chronic disease
Advancement in Technology
Regulatory Challenges in Japan's IVD Market
Request Free Customization of this report to help us to meet your business objectives.
To stay ahead in the in-vitro diagnostics market, key player focus on innovation, and rapid diagnostics solutions. The market is highly fragmented, with a few multinational corporations holding a substantial share, while many smaller and regional players compete in specialized segments. Roche Diagnostics leads the global in-vitro diagnostics market, driven by its extensive test portfolio, advanced technological capabilities, and strong global distribution network.
SkyQuest’s ABIRAW (Advanced Business Intelligence, Research & Analysis Wing) is our Business Information Services team that Collects, Collates, Correlates, and Analyses the Data collected using Primary Exploratory Research backed by robust Secondary Desk research.
According to SkyQuest’s analysis, the global In-Vitro Diagnostics (IVD) market is poised for sustained growth, fueled by the rising prevalence of chronic and infectious diseases, increasing healthcare awareness, and continuous advancements in diagnostic technologies. The adoption of AI and machine learning is enhancing diagnostic accuracy and enabling more personalized treatments. Point-of-care testing is gaining momentum, particularly in remote and underserved regions, due to its quick turnaround time and ease of use. Additionally, there is a noticeable shift toward home-based testing, driven by consumer demand for convenience and faster results. Immunoassays and molecular diagnostics are leading the market owing to their high sensitivity and specificity, especially in oncology and infectious disease detection. Asia-Pacific is emerging as a high-growth region due to improving healthcare infrastructure and supportive policies. Despite challenges like regulatory hurdles and reimbursement limitations, innovation, digitalization, and strategic partnerships are expected to drive the continued expansion of the IVD market.
| Report Metric | Details |
|---|---|
| Market size value in 2024 | USD 111.19 Billion |
| Market size value in 2033 | USD 163.82 Billion |
| Growth Rate | 4.4% |
| Base year | 2024 |
| Forecast period | 2026–2033 |
| Forecast Unit (Value) | USD Billion |
| Segments covered |
|
| Regions covered | North America (US, Canada), Europe (Germany, France, United Kingdom, Italy, Spain, Rest of Europe), Asia Pacific (China, India, Japan, Rest of Asia-Pacific), Latin America (Brazil, Rest of Latin America), Middle East & Africa (South Africa, GCC Countries, Rest of MEA) |
| Companies covered |
|
| Customization scope | Free report customization with purchase. Customization includes:-
|
To get a free trial access to our platform which is a one stop solution for all your data requirements for quicker decision making. This platform allows you to compare markets, competitors who are prominent in the market, and mega trends that are influencing the dynamics in the market. Also, get access to detailed SkyQuest exclusive matrix.
Table Of Content
Executive Summary
Market overview
Parent Market Analysis
Market overview
Market size
KEY MARKET INSIGHTS
COVID IMPACT
MARKET DYNAMICS & OUTLOOK
Market Size by Region
KEY COMPANY PROFILES
Methodology
For the In-Vitro Diagnostics Market, our research methodology involved a mixture of primary and secondary data sources. Key steps involved in the research process are listed below:
1. Information Procurement: This stage involved the procurement of Market data or related information via primary and secondary sources. The various secondary sources used included various company websites, annual reports, trade databases, and paid databases such as Hoover's, Bloomberg Business, Factiva, and Avention. Our team did 45 primary interactions Globally which included several stakeholders such as manufacturers, customers, key opinion leaders, etc. Overall, information procurement was one of the most extensive stages in our research process.
2. Information Analysis: This step involved triangulation of data through bottom-up and top-down approaches to estimate and validate the total size and future estimate of the In-Vitro Diagnostics Market.
3. Report Formulation: The final step entailed the placement of data points in appropriate Market spaces in an attempt to deduce viable conclusions.
4. Validation & Publishing: Validation is the most important step in the process. Validation & re-validation via an intricately designed process helped us finalize data points to be used for final calculations. The final Market estimates and forecasts were then aligned and sent to our panel of industry experts for validation of data. Once the validation was done the report was sent to our Quality Assurance team to ensure adherence to style guides, consistency & design.
Analyst Support
Customization Options
With the given market data, our dedicated team of analysts can offer you the following customization options are available for the In-Vitro Diagnostics Market:
Product Analysis: Product matrix, which offers a detailed comparison of the product portfolio of companies.
Regional Analysis: Further analysis of the In-Vitro Diagnostics Market for additional countries.
Competitive Analysis: Detailed analysis and profiling of additional Market players & comparative analysis of competitive products.
Go to Market Strategy: Find the high-growth channels to invest your marketing efforts and increase your customer base.
Innovation Mapping: Identify racial solutions and innovation, connected to deep ecosystems of innovators, start-ups, academics, and strategic partners.
Category Intelligence: Customized intelligence that is relevant to their supply Markets will enable them to make smarter sourcing decisions and improve their category management.
Public Company Transcript Analysis: To improve the investment performance by generating new alpha and making better-informed decisions.
Social Media Listening: To analyze the conversations and trends happening not just around your brand, but around your industry as a whole, and use those insights to make better Marketing decisions.
REQUEST FOR SAMPLE
Molecular diagnostics account for about 30–32% of total IVD revenue in 2024, growing fastest due to infectious-disease and genetic testing demand.
POC testing contributes approximately USD 35 billion, or 30% of total IVD revenue, with significant adoption in home and emergency settings.
Over 3 billion IVD tests are conducted annually worldwide, including clinical chemistry, immunoassay, and molecular tests.
Molecular diagnostics and next-generation sequencing (NGS) are expanding at a CAGR of 10–12%, driven by precision medicine.
Diagnostics account for about 2–3% of total healthcare spending, yet influence 60–70% of clinical decisions.
In 2024, investments in AI-enabled IVD technologies exceeded USD 2.5 billion, reflecting growing automation and analytics capabilities.
More than 1 million analyzers were installed globally by 2024, with a steady 4–5% annual increase driven by laboratory automation.
Global In-Vitro Diagnostics Market size was valued at USD 111.19 Billion in 2024 and is poised to grow from USD 116.08 Billion in 2025 to USD 163.82 Billion by 2033, growing at a CAGR of 4.4% during the forecast period (2026–2033).
F. Hoffmann-La Roche Ltd, Hologic Inc, Abbott Laboratories, Siemens Healthcare GmbH (Siemens Healthineers), Exact Sciences Corp, bioMérieux SA, QuidelOrtho Corp, Sysmex Corp, Bio-Rad Laboratories Inc, Thermo Fisher Scientific Inc, Becton Dickinson, Danaher Corporation (Beckman Coulter, Cepheid etc.), Illumina, DiaSorin (Luminex Corporation), Koninklijke Philips NV (Philips Healthcare), Qiagen N.V., Werfen, S.A. (Biokit S.A.), Agilent Technologies Inc, Quest Diagnostics, Revvity Inc, Mindray
The key driver of the in-vitro diagnostics (IVD) market is the increasing demand for early and accurate disease detection, monitoring, and personalized treatment, fueled by rising prevalence of chronic diseases, technological advancements, and growing adoption of diagnostic testing in healthcare systems.
A key market trend in the in-vitro diagnostics (IVD) market is the growing adoption of molecular diagnostics, point-of-care testing, and AI-driven diagnostic platforms, enabling faster, more accurate, and personalized disease detection and patient management.
North America accounted for the largest share in the in-vitro diagnostics (IVD) market, driven by advanced healthcare infrastructure, high adoption of diagnostic technologies, strong R&D investments, and the presence of leading IVD companies.
Want to customize this report? This report can be personalized according to your needs. Our analysts and industry experts will work directly with you to understand your requirements and provide you with customized data in a short amount of time. We offer $1000 worth of FREE customization at the time of purchase.
Feedback From Our Clients