
Report ID: UCMIG35J2144
SkyQuest Technology's Bioequivalence studies market size, share and forecast Report is based on the analysis of market data and Industry trends impacting the global Bioequivalence Studies Market and the revenue of top companies operating in it. Market Size Data and Statistics are based on the comprehensive research by our Team of Analysts and Industry experts.
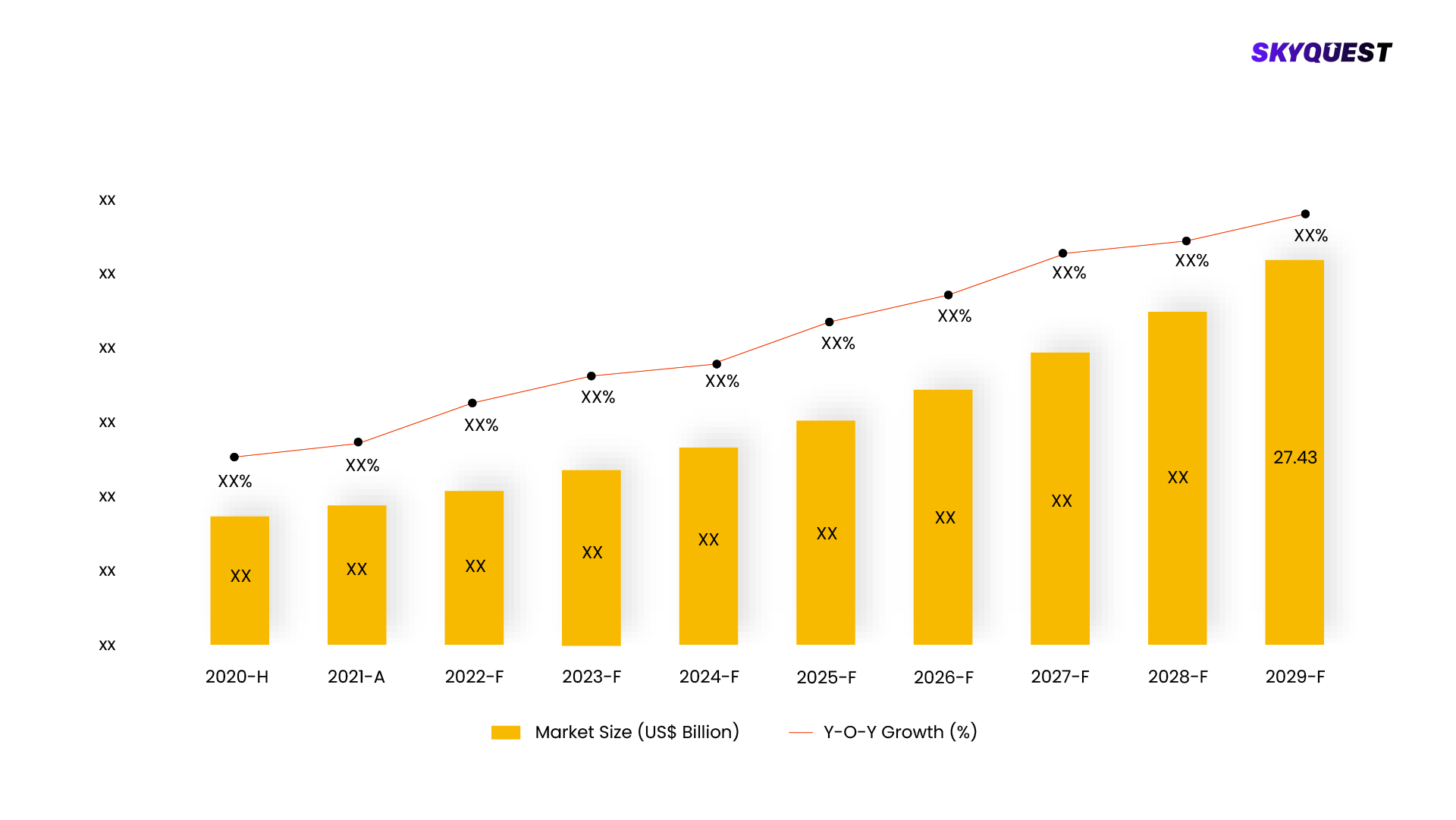
In 2022, the global market for bioequivalence studies had a total worth of USD 679.8 million, and it is projected to experience an annual growth rate of 8.3% from 2023 to 2030. One of the primary drivers fueling this growth is the rising demand for generic and biosimilar medications. Bioequivalence studies play a vital role in the development of pharmaceutical and biopharmaceutical products by comparing two formulations of the same drug or two different drug products to determine if they exhibit similar rates of bioavailability and pharmacokinetic parameters. These studies are commonly conducted for generic drugs, biosimilars, or when there are modifications to a drug formulation during its development process.
This report is being written to illustrate the market opportunity by region and by segments, indicating opportunity areas for the vendors to tap upon. To estimate the opportunity, it was very important to understand the current market scenario and the way it will grow in future.
Production and consumption patterns are being carefully compared to forecast the market. Other factors considered to forecast the market are the growth of the adjacent market, revenue growth of the key market vendors, scenario-based analysis, and market segment growth.
The market size was determined by estimating the market through a top-down and bottom-up approach, which was further validated with industry interviews. Considering the nature of the market we derived the Life Sciences Tools & Services by segment aggregation, the contribution of the Life Sciences Tools & Services in Pharmaceuticals, Biotechnology & Life Sciences and vendor share.
To determine the growth of the market factors such as drivers, trends, restraints, and opportunities were identified, and the impact of these factors was analyzed to determine the market growth. To understand the market growth in detail, we have analyzed the year-on-year growth of the market. Also, historic growth rates were compared to determine growth patterns.
REQUEST FOR SAMPLE
Want to customize this report? This report can be personalized according to your needs. Our analysts and industry experts will work directly with you to understand your requirements and provide you with customized data in a short amount of time. We offer $1000 worth of FREE customization at the time of purchase.
Feedback From Our Clients

Report ID: UCMIG35J2144
sales@skyquestt.com
USA +1 351-333-4748