
Report ID: SQMIG35D2329

Report ID: SQMIG35D2329
sales@skyquestt.com
USA +1 351-333-4748

Report ID:
SQMIG35D2329 |
Region:
Global |
Published Date: July, 2025
Pages:
197
|Tables:
111
|Figures:
68



Global Point of Care Diagnostics Market size was valued at USD 47.4 Billion in 2024 and is poised to grow from USD 50.01 Billion in 2025 to USD 76.75 Billion by 2033, growing at a CAGR of 5.5% during the forecast period (2026–2033).
Surge in prevalence of infectious diseases, rising burden of chronic diseases around the world, adoption of home-based and decentralized care, availability of favorable policies, and emphasis on patient empowerment are boosting sales of point of care diagnostics solutions.
A consumer‑driven movement towards self‑care is primarily bolstering point of care diagnostics market growth. Governments and healthcare programs are promoting decentralized care models, further driving integration of point of care diagnostics into primary care and home settings. A robust increase in geriatric population base coupled with chronic disease incidence like diabetes, cardiovascular disorders, and cancer is driving demand for continuous, decentralized monitoring. POC diagnostics for glucose, lipids, cardiac markers, and biomarkers enable rapid, on‑site testing that supports proactive disease management. The global increase in infectious diseases like COVID‑19, HIV, tuberculosis, and malaria has significantly accelerated demand for rapid, on‑site diagnostic testing, thereby boosting popularity of point of care diagnostics.
On the contrary, high costs of advanced point of care devices, complexity in regulations and reimbursement policies, variability in accuracy, and limited technical expertise in developing regions are estimated to limit the global point of care diagnostics market penetration through 2032 and beyond.
How Digital Health Integration Transforming Point of Care Diagnostics?
The integration of point of care diagnostics with digital health technologies is revolutionizing patient monitoring and care delivery. Many modern point of care devices is now Bluetooth-enabled and compatible with smartphones or tablets, allowing real-time transmission of diagnostic data to healthcare providers. Integration with telemedicine platforms enhances remote patient management, especially for chronic illnesses and infectious diseases. AI-powered algorithms are also improving test interpretation and reducing human error. This digital synergy enhances personalized treatment plans, accelerates clinical decisions, and strengthens continuity of care. As healthcare increasingly shifts toward remote and value-based models, digital integration is becoming central point of care diagnostics in the future landscape.
To get more insights on this market click here to Request a Free Sample Report
Global point of care diagnostics market is segmented by product, sample, end user, and region. Based on product, the market is segmented into glucose testing, Hb1Ac testing, coagulation testing, fertility/pregnancy, infectious disease, cardiac markers, thyroid stimulating hormone, hematology, primary care systems, decentralized clinical chemistry, feces, lipid testing, cancer marker, blood gas/electrolytes, ambulatory chemistry, drug of abuse (DOA) testing, autoimmune diseases, urinalysis/nephrology. Based on samples, the market is segmented into blood, nasal and oropharyngeal swabs, urine, and other factors. Based on end user, the market is segmented into clinics (pharmacy & retail clinics, physician office, urgent care clinics, non-practice clinics), hospitals, homes, assisted living healthcare facilities, and laboratories. Based on region, the market is segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
Which Point of Care Diagnostics Product is Slated to Bring in the Most Revenue?
The infectious disease segment is estimated to hold the highest global point of care diagnostics market share going forward. The surge in demand for rapid testing of infectious diseases and emphasis on improving infectious disease management are helping this segment maintain a dominant stance. Government efforts to restrict the rising prevalence of multiple types of infectious diseases are also helping this segment hold sway over others.
On the other hand, the demand for cancer markets is expected to rise at a robust pace over the coming years as per this point of care diagnostics market forecast. Growing incidence of cancer around the world and growing emphasis on early detection of cancer are helping make this a highly opportune segment in the future.
Where are Most Point of Care diagnostics Solutions Used?
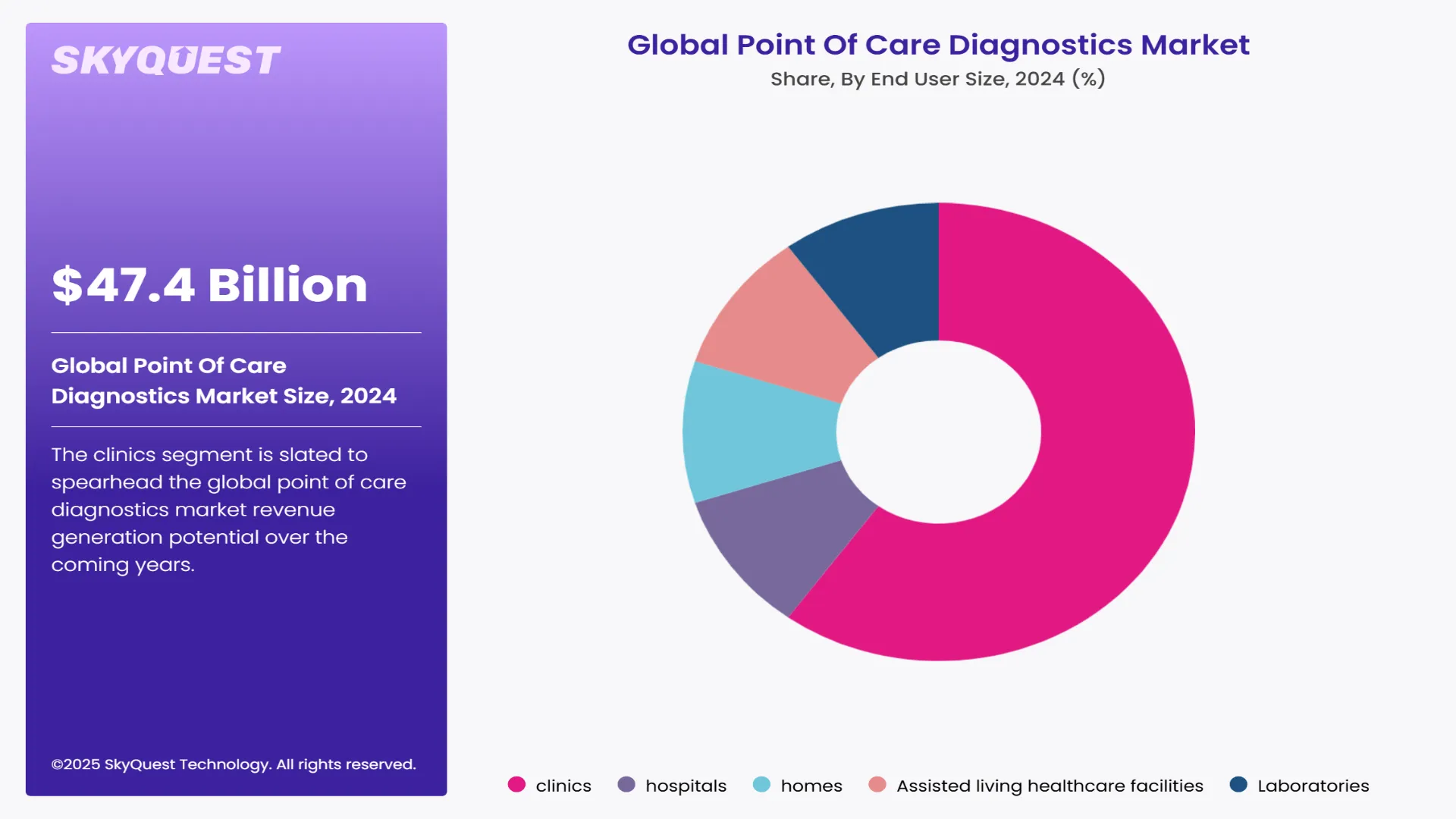
The clinics segment is slated to spearhead the global point of care diagnostics market revenue generation potential over the coming years. Professionals working in clinics are shifting towards point of care diagnostics from conventional lab testing to help shorten testing time and improve patient outcomes. Low costs and easy availability of point of care diagnostics tests are also helping boost their adoption in clinics.
Meanwhile, the demand for point of care diagnostics in the home segment is slated to rise at a notable pace over the coming years. Rising popularity of homecare around the world and increasing preference for the same by geriatric population are helping create new opportunities via this segment.
To get detailed segments analysis, Request a Free Sample Report
Why is Adoption of Point of Care Diagnostics High in North America?
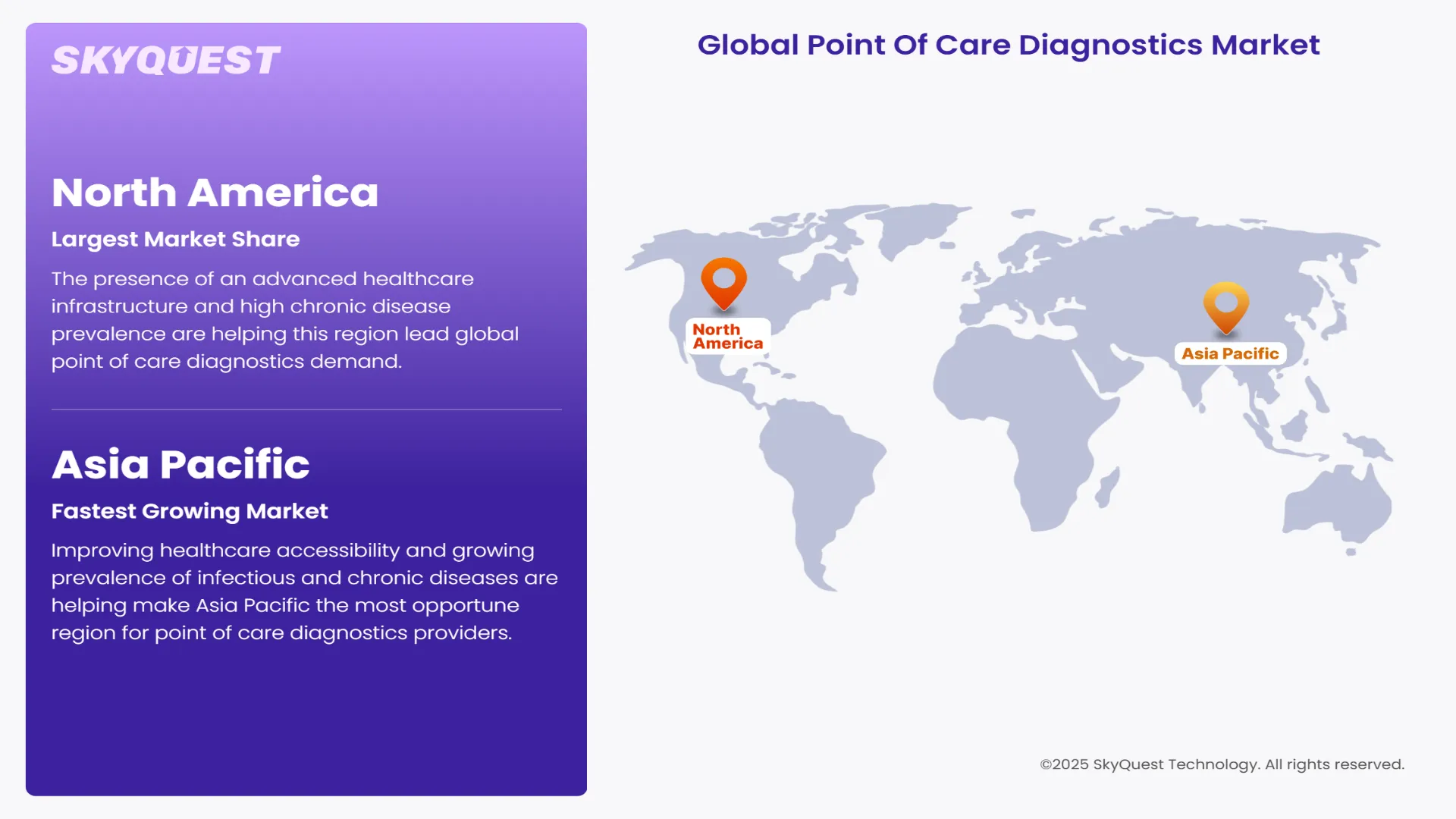
The presence of an advanced healthcare infrastructure and high chronic disease prevalence are helping this region lead global point of care diagnostics demand. Early adoption of innovative point of care diagnostic technologies, government funding, and favorable reimbursement frameworks are also forecasted to help this region maintain its high share over the coming years. Consumer familiarity with self-testing devices and surge in popularity of home healthcare are also estimated to boost revenue generation potential of this region through 2032.
Point Of Care Diagnostics Market in United States
The surge in demand for rapid testing and presence of a well-developed healthcare infrastructure make this country the largest market for point of care diagnostics providers. Presence of top point of care diagnostics companies such as Abbott, BD, and Quidel also helps the United States hold sway over other countries. CLIA-waived tests enable decentralized diagnostics in clinics, pharmacies, and home settings. Adoption of telehealth and digital platforms is also estimated to create new opportunities in the long run.
Point of Care Diagnostics Market in Canada
A shift towards decentralized care and emphasis on improving healthcare access in rural areas are promoting point of care diagnostics adoption in Canada. Health Canada regulates and approves home-use and clinical POC tests with increasing efficiency Strong emphasis on patient-centric care is also anticipated to create new business scope for point of care diagnostics companies. Canada's point of care diagnostics market also benefits from collaborations with manufacturers from the United States and local innovation.
Why are Point of Care Diagnostics Innovators Targeting Asia Pacific?
Improving healthcare accessibility and growing prevalence of infectious and chronic diseases are helping make Asia Pacific the most opportune region for point of care diagnostics providers. Robust investments in rural healthcare infrastructure development and telemedicine adoption are also projected to boost point of care diagnostics demand in countries such as South Korea, China, Japan, Indonesia, and India going forward. The shift toward decentralized testing in resource-limited settings and rising demand for self-diagnostic tools are forecasted to ensure a sustained demand for novel point of care diagnostics solutions.
Point of Care Diagnostics Market in Japan
Growing incidence of lifestyle-related diseases such as diabetes and cardiovascular conditions coupled with a massive geriatric population make Japan a key market for point of care diagnostics providers. Demand for advanced molecular and immunoassay-based devices is high among the Japanese population. Domestic companies like Sysmex and Nipro are also playing a crucial role in driving innovation in point of care diagnostics. Cultural preference for preventive healthcare further boosts home and clinic-based POC testing.
Point of Care Diagnostics Market in South Korea
Launch of new government health initiatives and presence of a digitally savvy population are helping boost point of care diagnostics demand in South Korea. Strong national health insurance and proactive disease screening programs support diagnostic adoption. Integration of point of care diagnostics with mobile health and telemedicine platforms is expected to be a popular trend in the long run. South Korea’s innovation ecosystem facilitates collaboration between academia, industry, and government.
Should Point of Care Diagnostics Companies Invest in Europe?
The presence of strong public healthcare systems and growing emphasis on preventive healthcare are estimated to bolster the sales of point of care diagnostics solutions in Europe. Regulatory support and reimbursement for home-based diagnostics are also predicted to create new opportunities for point of care diagnostics companies in the long run. Cross-border collaborations and government funding are slated to play a crucial role in advancing the point of care diagnostics innovation over the coming years.
Point of Care Diagnostics Market in United Kingdom
NHS-led digital transformation and growing emphasis on chronic disease management are expected to govern point of care diagnostics demand in the United Kingdom. Incorporation of point of care testing into primary care for managing diabetes, infections, and cardiovascular risks is also helping expand revenue generation for market players. Strategic partnerships, academic collaborations, and government funding are further creating new business scope for market players operating in the country.
Point of Care Diagnostics Market in Germany
Presence of a developed healthcare infrastructure and government emphasis on preventive care are forecasted to make Germany a key market for point of care diagnostics providers. The senescent population of the country is driving up the demand for advanced and quick point of care diagnostics. Germany’s decentralized healthcare model favors adoption of rapid testing in pharmacies and general practices. The presence of leading diagnostics companies such as Roche and Siemens is also helping create new opportunities in the country.
Point of Care Diagnostics Market in France
Rising emphasis on early detection of diseases and improvement of outpatient care are helping boost point of care diagnostics adoption in the country. The Assurance Maladie national insurance system supports partial reimbursement for approved POC tests, improving accessibility. France’s investment in e-health infrastructure, combined with an increasing emphasis on patient autonomy and home-based care, strengthens the POC diagnostics ecosystem nationwide.
To know more about the market opportunities by region and country, click here to
Buy The Complete Report
Point of Care Diagnostics Market Drivers
Advancements in Point of Care Diagnostics Technologies
Availability of Favorable Policies and Funding
Point of Care Diagnostics Market Restraints
Variability in Accuracy and Quality Assurance
High Costs of Advanced Devices and Consumables
Request Free Customization of this report to help us to meet your business objectives.
Point of care diagnostics providers should focus on increasing the efficacy of their tests and reduce their result times by investing in R&D. Developing at-home and rapid POC test kits is also a prominent opportunity for companies as per this global point of care diagnostics market analysis. Targeting countries with developed healthcare infrastructure is also a key strategy for all market players.
Reducing the costs and results of novel point of care diagnostics is slated be the prime focus of new companies. Here’s a startup that has the potential to augment the point of care diagnostics industry in the long run.
Top Player’s Company Profiles
Recent Developments in Point of Care Diagnostics Market
SkyQuest’s ABIRAW (Advanced Business Intelligence, Research & Analysis Wing) is our Business Information Services team that Collects, Collates, Correlates, and Analyses the Data collected by means of Primary Exploratory Research backed by robust Secondary Desk research.
As per SkyQuest analysis, the growing prevalence of chronic diseases and advancements in point of care diagnostics technologies are anticipated to drive the demand for point of care diagnostics going forward. However, high costs of devices and variability in accuracy are slated to slow down the adoption of point of care diagnostics in the future. North America is slated to spearhead the demand for point of care diagnostics owing to the presence of a developed healthcare infrastructure and favorable reimbursement policies. The development of at-home testing kits and expansion of molecular POC testing are anticipated to be key trends driving the point of care diagnostics industry through 2032 and beyond.
| Report Metric | Details |
|---|---|
| Market size value in 2024 | USD 47.4 Billion |
| Market size value in 2033 | USD 76.75 Billion |
| Growth Rate | 5.5% |
| Base year | 2024 |
| Forecast period | 2026-2033 |
| Forecast Unit (Value) | USD Billion |
| Segments covered |
|
| Regions covered | North America (US, Canada), Europe (Germany, France, United Kingdom, Italy, Spain, Rest of Europe), Asia Pacific (China, India, Japan, Rest of Asia-Pacific), Latin America (Brazil, Rest of Latin America), Middle East & Africa (South Africa, GCC Countries, Rest of MEA) |
| Companies covered |
|
| Customization scope | Free report customization with purchase. Customization includes:-
|
To get a free trial access to our platform which is a one stop solution for all your data requirements for quicker decision making. This platform allows you to compare markets, competitors who are prominent in the market, and mega trends that are influencing the dynamics in the market. Also, get access to detailed SkyQuest exclusive matrix.
Table Of Content
Executive Summary
Market overview
Parent Market Analysis
Market overview
Market size
KEY MARKET INSIGHTS
COVID IMPACT
MARKET DYNAMICS & OUTLOOK
Market Size by Region
KEY COMPANY PROFILES
Methodology
For the Point Of Care Diagnostics Market, our research methodology involved a mixture of primary and secondary data sources. Key steps involved in the research process are listed below:
1. Information Procurement: This stage involved the procurement of Market data or related information via primary and secondary sources. The various secondary sources used included various company websites, annual reports, trade databases, and paid databases such as Hoover's, Bloomberg Business, Factiva, and Avention. Our team did 45 primary interactions Globally which included several stakeholders such as manufacturers, customers, key opinion leaders, etc. Overall, information procurement was one of the most extensive stages in our research process.
2. Information Analysis: This step involved triangulation of data through bottom-up and top-down approaches to estimate and validate the total size and future estimate of the Point Of Care Diagnostics Market.
3. Report Formulation: The final step entailed the placement of data points in appropriate Market spaces in an attempt to deduce viable conclusions.
4. Validation & Publishing: Validation is the most important step in the process. Validation & re-validation via an intricately designed process helped us finalize data points to be used for final calculations. The final Market estimates and forecasts were then aligned and sent to our panel of industry experts for validation of data. Once the validation was done the report was sent to our Quality Assurance team to ensure adherence to style guides, consistency & design.
Analyst Support
Customization Options
With the given market data, our dedicated team of analysts can offer you the following customization options are available for the Point Of Care Diagnostics Market:
Product Analysis: Product matrix, which offers a detailed comparison of the product portfolio of companies.
Regional Analysis: Further analysis of the Point Of Care Diagnostics Market for additional countries.
Competitive Analysis: Detailed analysis and profiling of additional Market players & comparative analysis of competitive products.
Go to Market Strategy: Find the high-growth channels to invest your marketing efforts and increase your customer base.
Innovation Mapping: Identify racial solutions and innovation, connected to deep ecosystems of innovators, start-ups, academics, and strategic partners.
Category Intelligence: Customized intelligence that is relevant to their supply Markets will enable them to make smarter sourcing decisions and improve their category management.
Public Company Transcript Analysis: To improve the investment performance by generating new alpha and making better-informed decisions.
Social Media Listening: To analyze the conversations and trends happening not just around your brand, but around your industry as a whole, and use those insights to make better Marketing decisions.
REQUEST FOR SAMPLE
Want to customize this report? This report can be personalized according to your needs. Our analysts and industry experts will work directly with you to understand your requirements and provide you with customized data in a short amount of time. We offer $1000 worth of FREE customization at the time of purchase.
Feedback From Our Clients