
Report ID: SQMIG35H2346

Report ID: SQMIG35H2346
sales@skyquestt.com
USA +1 351-333-4748

Report ID:
SQMIG35H2346 |
Region:
Global |
Published Date: August, 2025
Pages:
191
|Tables:
115
|Figures:
70



Global Circulating Tumor Cell Market size was valued at USD 13.03 Billion in 2024 and is poised to grow from USD 14.28 Billion in 2025 to USD 29.74 Billion by 2033, growing at a CAGR of 9.6% in the forecast period (2026–2033).
The global circulating tumor cell market growth is driven due to their advantages and noninvasiveness. This is the promising tool in the diagnosis of cancer. The growing global incidence of cancer is one of the primary factors driving the market's growth. The Pan American Health Organization estimates that in 2023, there were about 20 million new cases of cancer and 10 million deaths from the disease. Over the next 20 years, the cancer burden is predicted to rise by 60%, posing new challenges for individuals, communities, and healthcare systems. Globally, the incidence of cancer is estimated to reach 30 million new cases by 2040.
The global circulating tumor cell industry is being fueled by demand for less invasive methodologies for cancer monitoring and detection of "cancer" by testing blood samples. Therefore, liquid biopsy technologies are gaining traction around the world, facilitating the monitoring of tumor dynamics and treatment response in real time. In July 2024, Bio-Rad Laboratories, Inc. unveiled Celselect Slides 2.0, to improve the capture of rare cells and circulating tumor cells (CTCs), as well as processing larger volumes of liquid biopsy samples for counting or beyond.
By increasing detection sensitivity, automating image analysis, and enabling predictive oncology insights, artificial intelligence (AI) is significantly influencing the global circulating tumor cell (CTC) market outlook. By more accurately distinguishing CTCs from healthy blood cells, sophisticated machine learning techniques can lower false positives. RareCyte and Microsoft Azure teamed up in 2024 to integrate AI-powered image pipelines and expedite cancer detection processes. AI-powered platforms also facilitate long-term tracking of metastasis progression, enabling real-time treatment customization by oncologists.
To get more insights on this market click here to Request a Free Sample Report
The global circulating tumor cell market is segmented into technology, product, specimen, end user, and region. By technology, the market is classified into circulating tumor cells CTC detection & enrichment methods, CTC direct detection methods, and CTC analysis. Depending on the product, it is divided into kits & reagents, blood collection tubes, and devices or systems. According to the specimen, the market is bifurcated into blood and bone marrow. As per end user, it is categorized into research & academic institutes, hospital & clinics, and diagnostic centers. Regionally, it is analyzed across North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa.
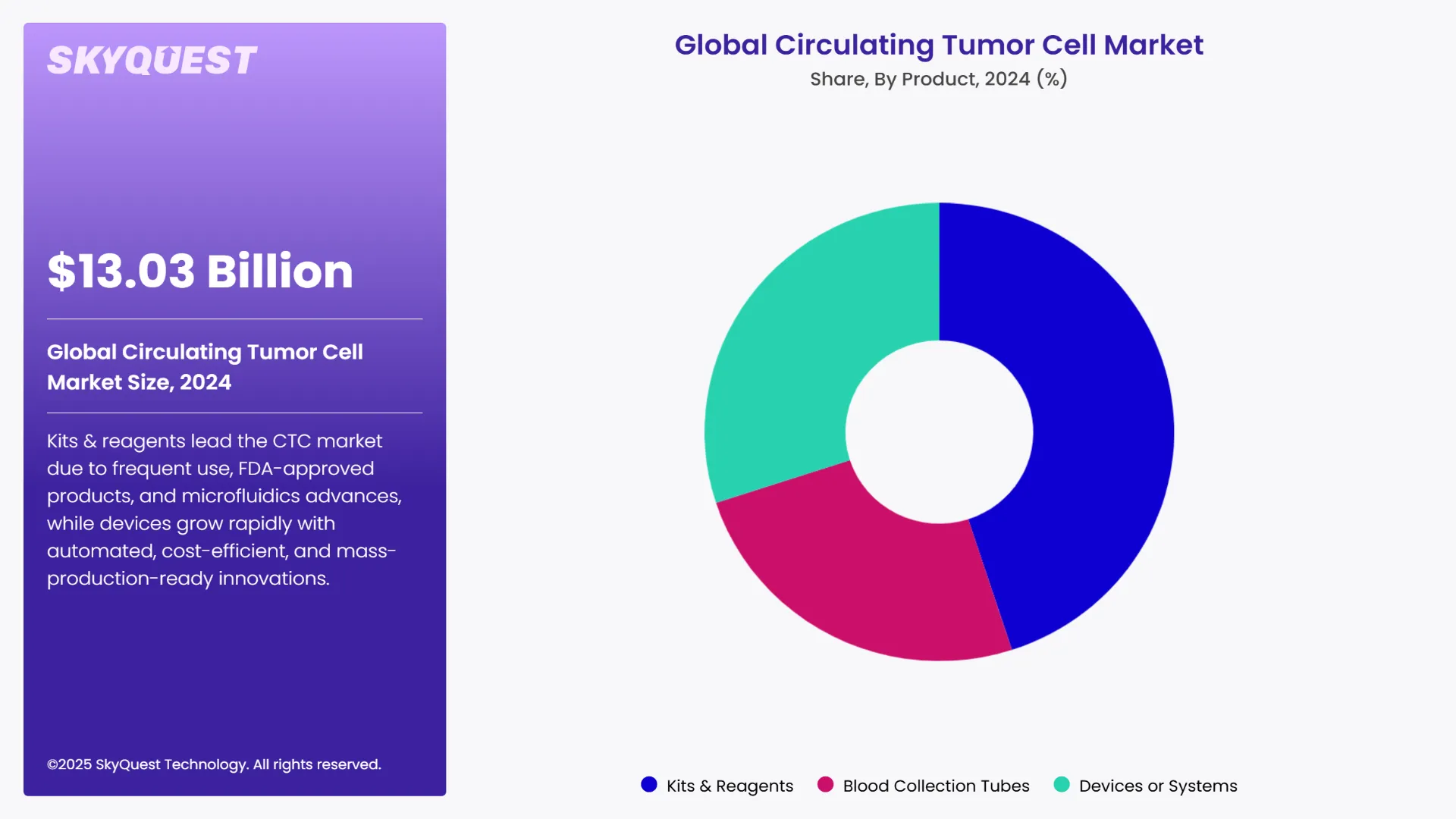
As per the 2024 circulating tumor cell market analysis, the kits & reagents segment led the market with the largest revenue share of 45.34%. This is caused by frequent purchases and high usage rates. For instance, the FDA-approved CellSearch Circulating Tumour Cell Kit is among the most well-known goods produced in the US. Additionally, advancements in microfluidics technology and the availability of a robust product portfolio are expected to fuel market growth.
The devices or systems segment is expected to have the largest circulating tumor cell market share during the forecast period. By overcoming the challenges and enhancing technical completeness for mass production, the introduction of fabricated glass microchips is expected to propel the segment's growth. The development of automated devices that eliminate the need for additional tubes lowers the cost of blood collection tubes.
As per the 2024 circulating tumor cell market forecast, the blood category led the market with the largest revenue share of 48.13%. Compared to other biospecimens, blood samples have a higher concentration of these cells, which results in the specimen type's greatest penetration. In today's cancer research, techniques for detecting tumor cells in blood samples are considered essential because they aid in prognostic prediction and evaluation of the effectiveness of systemic chemotherapy. However, there is a problem when using whole blood as a specimen in conjunction with microfluidic technology.
The bone marrow segment is expected to grow at a notable compound annual growth rate during the forecast period. Bone marrow samples that have been tested for CTCs are crucial for cancer research and clinical trials. Research scientists use these samples to study metastasis processes, study the physiology of circulating tumor cells, and devise new treatment methods. All of these aspects ought to drive segment growth during the forecast period.
To get detailed segments analysis, Request a Free Sample Report
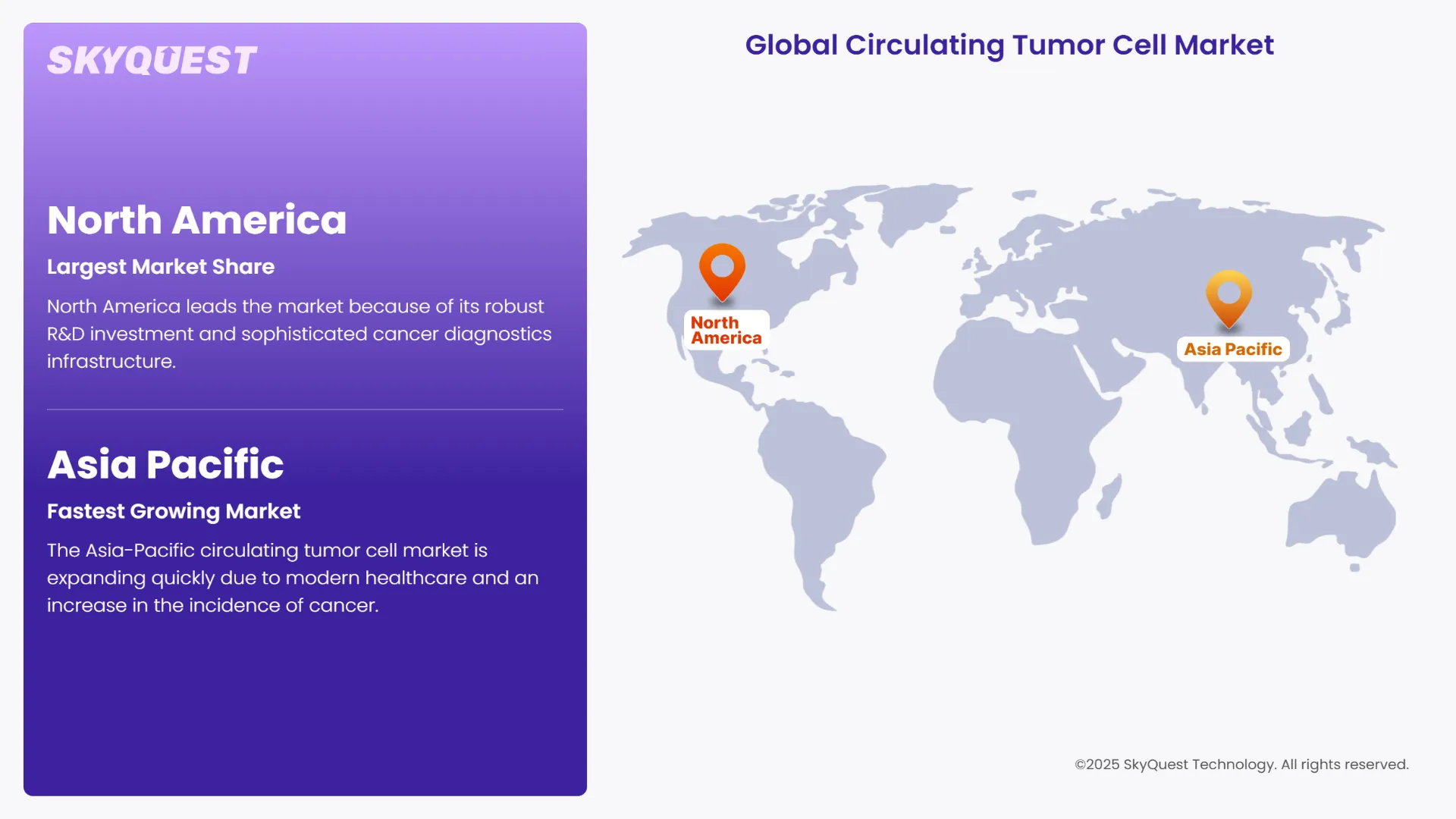
As per the circulating tumor cell market regional analysis, North America leads the market because of its robust R&D investment and sophisticated cancer diagnostics infrastructure. The clinical implementation of AI-integrated CTC analysis platforms in the region was expedited in 2024 by FDA clearances. Innovation was further stimulated by partnerships between academic cancer centers and liquid biopsy developers. The US is expected to witness over 2 million more cases of cancer in 2025, which increases the need for early detection tools and positions North America as a center for the growth of precision oncology.
US Circulating Tumor Cell Market
The US leads the CTC research due to significant funding from organizations like the National Cancer Institute. Menarini Silicon Biosystems improved metastatic cancer surveillance in 2024 by expanding its CELLSEARCH platform trials with major hospitals. The use of liquid biopsy instruments is being driven by the increased incidence of prostate and breast cancers. Next-generation CTC detection technologies can be more rapidly incorporated into routine oncology diagnostics across the country with expanded healthcare coverage and regulatory support.
Canada Circulating Tumor Cell Market
Government-funded cancer research projects and growing precision medicine initiatives are the main drivers of Canada's circulating tumor cell sector expansion. The Princess Margaret Cancer Centre began trials for AI-powered CTC analysis for early-stage breast cancer in 2024. There is an increasing impetus for minimally invasive diagnostics, particularly due to increasing colorectal cancer rates which are expected to increase by over 20% in those under 50 by 2025. There are national health policies in support of liquid biopsy procedures, including recognition of CTC technologies as essential components for individualized treatment decisions.
The CTC market in Europe continues to be robust, buoyed by a supportive regulatory environment and established cancer screening programs. As an example, in 2024 the European Commission analyzed several multi-country studies looking at CTC enumeration as a measure of treatment response in lung cancer. Countries like Germany, France and the UK all have strong biotech hubs established for CTC isolation technology and are now emphasizing the integration of liquid biopsy data into patient management systems. CTC adoption will largely depend on the ongoing dynamics in relationships between hospitals and device manufacturers.
UK Circulating Tumor Cell Market
The NHS Genomic Medicine Service's incorporation of liquid biopsy trials into cancer care pathways is advantageous for the UK's CTC market. Researchers from Angle plc and Oxford University partnered in 2024 to enhance CTC capture for ovarian cancer screening. With rising cancer rates, predicted to reach 4 million cases by 2025, interest in CTC capture is growing. Advances in the use of complex platforms for dedifferentiated CTC analysis for clinical use, along with strong funding in the biotech sector and the government's focus on early diagnosis, are driving this interest.
France Circulating Tumor Cell Market
In France, national cancer control programs are pushing the CTC market from prioritization of precision therapy and early diagnosis. In 2024, Institut Gustave Roussy initiated a liquid biopsy program using CTC profiling to guide metastatic breast cancer therapy. The CTC market is also benefiting from improving accessibility with collaboration between biotech companies and public hospitals and reimbursement policies that promote CTC use. The nation's oncology networks are using non-invasive CTC-based diagnosis as the incidence of lung and colorectal cancer rises.
Germany Circulating Tumor Cell Market
Germany is at the leading edge of CTC adoption in Europe due to its developed networks for undertaking, clinical trials of CTCs and growing biotech industry. To meet increased demand from customers, Qiagen expanded its Hannover facility in 2024, to produce the next generation CTCs isolation kits. Investment is being increased in liquid biopsy research here, at least in part because of the similar high incidence of cancer in general, particularly breast cancer and prostate cancer. Precision oncology programs in the country formed with university-hospitals have stimulated work around undertaking clinical applications of CTC analysis, including therapy monitoring and research usage.
The Asia-Pacific circulating tumor cell market is expanding quickly due to modern healthcare and an increase in the incidence of cancer. As AI-powered CTC analysis gained traction in China, South Korea, and Japan in 2024, regional governments boosted funding for liquid biopsy research and development. Technology transfer is accelerated through collaborations between multinational corporations and regional biotech companies. The market outlook for both emerging and developed economies is supported by the low cost of blood-based diagnostics and rising awareness of early cancer detection.
South Korea Circulating Tumor Cell Market
The circulating tumor cell market in South Korea is growing due to substantial government investment in precision oncology and biotech innovation, . In 2024, a clinical validation test was completed for a CTC detection platform for stomach cancer. This is a common disease in the country and was conducted by Seoul National University Hospital. The push for adoption is supported by collaboration with various global medtech partners and growing participation rates in cancer screening. The national policies on personalized cancer therapies currently include CTC testing as part of ongoing diagnostic routine to increase the circulating tumor cell market penetration.
Japan Circulating Tumor Cell Market
Japan's CTC industry benefits from a high incidence of breast, lung, and stomach cancers as well as a sophisticated medical research infrastructure. Sysmex Corporation and national cancer hospitals deployed an artificial intelligence-powered ctc enumeration system to improve detection accuracy beginning in 2024. The demand in the market is increasing and will continue to increase due to an aging population and large government initiatives to provide cancer screening to adults. CTC analysis is a predictive paradigm that fits with clinical decision making for early interventions which aligns with Japan's goal to precision medicine.
To know more about the market opportunities by region and country, click here to
Buy The Complete Report
Regulatory Body Clearances and Clinical Validation
Continuous Patient Monitoring and Precision Oncology
Sample Variability and Technical Constraints
Absence of Standard Practices and Financial Considerations
Request Free Customization of this report to help us to meet your business objectives.
While developing its cancer marker panels Menarini Silicon Biosystems keeps its market leadership with the CellSearch system. ANGLE plc, while working with pharmaceutical companies to conduct clinical trials with its FDA-cleared Parsortix system, also provides all the documentation needed for intensive research with validated quality assurance protocols. RareCyte provides protocols for in-depth research with validated quality control protocols. Epic Sciences has reimbursed an AR-V7 test for prostate cancer, focusing on a new, enriching free imaging technology that attracts physicians.
Recent Developments in Circulating Tumor Cell Market
SkyQuest’s ABIRAW (Advanced Business Intelligence, Research & Analysis Wing) is our Business Information Services team that Collects, Collates, Correlates, and Analyses the Data collected by means of Primary Exploratory Research backed by robust Secondary Desk research.
As per SkyQuest analysis, the circulating tumor cell market has moved from a research tool to a clinical application. Due to payor acceptance, regulatory approvals, and strong data supporting clinical benefit, there are compelling propositions for CTC testing to hospitals and pharma. However, there are challenges with standardization of the data and processes, obtaining all the CTC types a patient may uniquely shed with their cancer, and the expense. The technologies capable of addressing all tests and delivering comprehensive, automated analytics and objective data capture of viable CTCs should lead to continued growth. Due to these developments, CTC testing is now expected to be a valuable complement to other liquid biopsy modalities, particularly for minimal residual disease monitoring, therapy selection, and real-time assessment of treatment resistance.
| Report Metric | Details |
|---|---|
| Market size value in 2024 | USD 13.03 Billion |
| Market size value in 2033 | USD 29.74 Billion |
| Growth Rate | 9.6% |
| Base year | 2024 |
| Forecast period | 2026–2033 |
| Forecast Unit (Value) | USD Billion |
| Segments covered |
|
| Regions covered | North America (US, Canada), Europe (Germany, France, United Kingdom, Italy, Spain, Rest of Europe), Asia Pacific (China, India, Japan, Rest of Asia-Pacific), Latin America (Brazil, Rest of Latin America), Middle East & Africa (South Africa, GCC Countries, Rest of MEA) |
| Companies covered |
|
| Customization scope | Free report customization with purchase. Customization includes:-
|
To get a free trial access to our platform which is a one stop solution for all your data requirements for quicker decision making. This platform allows you to compare markets, competitors who are prominent in the market, and mega trends that are influencing the dynamics in the market. Also, get access to detailed SkyQuest exclusive matrix.
Table Of Content
Executive Summary
Market overview
Parent Market Analysis
Market overview
Market size
KEY MARKET INSIGHTS
COVID IMPACT
MARKET DYNAMICS & OUTLOOK
Market Size by Region
KEY COMPANY PROFILES
Methodology
For the Circulating Tumor Cell Market, our research methodology involved a mixture of primary and secondary data sources. Key steps involved in the research process are listed below:
1. Information Procurement: This stage involved the procurement of Market data or related information via primary and secondary sources. The various secondary sources used included various company websites, annual reports, trade databases, and paid databases such as Hoover's, Bloomberg Business, Factiva, and Avention. Our team did 45 primary interactions Globally which included several stakeholders such as manufacturers, customers, key opinion leaders, etc. Overall, information procurement was one of the most extensive stages in our research process.
2. Information Analysis: This step involved triangulation of data through bottom-up and top-down approaches to estimate and validate the total size and future estimate of the Circulating Tumor Cell Market.
3. Report Formulation: The final step entailed the placement of data points in appropriate Market spaces in an attempt to deduce viable conclusions.
4. Validation & Publishing: Validation is the most important step in the process. Validation & re-validation via an intricately designed process helped us finalize data points to be used for final calculations. The final Market estimates and forecasts were then aligned and sent to our panel of industry experts for validation of data. Once the validation was done the report was sent to our Quality Assurance team to ensure adherence to style guides, consistency & design.
Analyst Support
Customization Options
With the given market data, our dedicated team of analysts can offer you the following customization options are available for the Circulating Tumor Cell Market:
Product Analysis: Product matrix, which offers a detailed comparison of the product portfolio of companies.
Regional Analysis: Further analysis of the Circulating Tumor Cell Market for additional countries.
Competitive Analysis: Detailed analysis and profiling of additional Market players & comparative analysis of competitive products.
Go to Market Strategy: Find the high-growth channels to invest your marketing efforts and increase your customer base.
Innovation Mapping: Identify racial solutions and innovation, connected to deep ecosystems of innovators, start-ups, academics, and strategic partners.
Category Intelligence: Customized intelligence that is relevant to their supply Markets will enable them to make smarter sourcing decisions and improve their category management.
Public Company Transcript Analysis: To improve the investment performance by generating new alpha and making better-informed decisions.
Social Media Listening: To analyze the conversations and trends happening not just around your brand, but around your industry as a whole, and use those insights to make better Marketing decisions.
REQUEST FOR SAMPLE
Global Circulating Tumor Cell Market size was valued at USD 13.03 Billion in 2024 and is poised to grow from USD 14.28 Billion in 2025 to USD 29.74 Billion by 2033, growing at a CAGR of 9.6% in the forecast period (2026–2033).
While developing its cancer marker panels Menarini Silicon Biosystems keeps its market leadership with the CellSearch system. ANGLE plc, while working with pharmaceutical companies to conduct clinical trials with its FDA-cleared Parsortix system, also provides all the documentation needed for intensive research with validated quality assurance protocols. RareCyte provides protocols for in-depth research with validated quality control protocols. Epic Sciences has reimbursed an AR-V7 test for prostate cancer, focusing on a new, enriching free imaging technology that attracts physicians. 'QIAGEN', 'Bio-Techne Corporation', 'Ultima Genomics, Inc.', 'Bio-Rad Laboratories, Inc.', 'Dxcover Ltd.', 'Illumina, Inc.', 'Cell Microsystems', 'Greiner Bio One International GmbH', 'Ikonisys Inc.', 'Miltenyi Biotec', 'Creative Bioarray', 'BioFluidica', 'Abnova Corporation'
Strong clinical validation and regulatory approvals are driving the circulating tumor cell market. Research laboratories and medical facilities tend to prefer to use the approved platforms, such as ANGLE's Parsortix PC1 for harvesting CTCs and CellSearch for counting the CTCs. These platforms are receiving increasing use and collaborations with pharmaceutical companies because of the approvals and the ability for CTCs to assist in cancer prognosis and therapy selection.
One of the key circulating tumor cell industry trends include multi-omics and new capture methodologies. Numerous companies are creating innovative approaches to capture CTCs while not disrupting them in any way, including microfluidic devices. This provides scientists the opportunity to culture the cells, perform extensive genomic and proteomic studies, and do pharmacologic testing. The ability to culture the CTCs after capture provides a huge opportunity for personalized treatment options.
As per the circulating tumor cell market regional analysis, North America leads the market because of its robust R&D investment and sophisticated cancer diagnostics infrastructure. The clinical implementation of AI-integrated CTC analysis platforms in the region was expedited in 2024 by FDA clearances. Innovation was further stimulated by partnerships between academic cancer centers and liquid biopsy developers. The US is expected to witness over 2 million more cases of cancer in 2025, which increases the need for early detection tools and positions North America as a center for the growth of precision oncology.
Want to customize this report? This report can be personalized according to your needs. Our analysts and industry experts will work directly with you to understand your requirements and provide you with customized data in a short amount of time. We offer $1000 worth of FREE customization at the time of purchase.
Feedback From Our Clients